Why ATP? A Creation Model Perspective
There’s quite a buzz surrounding cryptocurrency these days. Plenty of people see investing in cryptocurrencies as a way to get rich quickly.
To be frank, I’m not sure I understand the ins and outs of cryptocurrency well enough to be comfortable investing in it.
Currency is a standardized form of exchange in an economy. It’s a universal store of value that can be presented or received in exchange for goods or services. The use of currency is so commonplace that it becomes easy to overlook its genius as a human invention and its critical importance to any economy. Without currency, we would all have to barter for goods and services. And bartering is extremely cumbersome and inefficient.
The Cell’s Energy Currency
Currency not only ensures the efficient operation of economies, but it also ensures the efficient operation of biochemical processes inside the cell. These processes require energy. And that energy is almost always supplied by the biomolecule, ATP (adenosine triphosphate)—a molecule dubbed the energy currency of the cell.
Biochemists have learned that for the cell’s biochemical processes to be suitably efficient, they require an energy currency molecule such as ATP. What’s the best explanation for how this indispensable energy currency emerged?
Broken Bonds Provide Energy
Let’s start with the structure of the biochemical energy source. ATP consists of an adenine attached to the sugar, ribose. Also bound to the ribose is a triphosphate group (see figure 1).
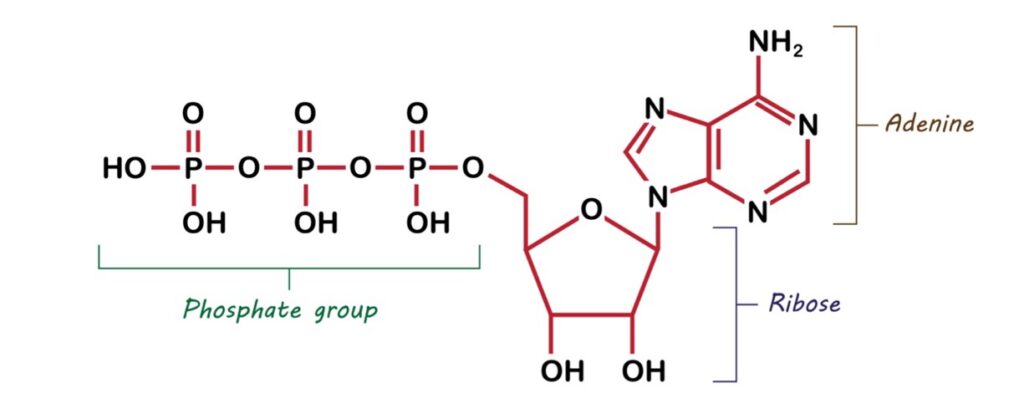
Figure 1: The Structure of ATP
Credit: Shutterstock
The triphosphate portion of ATP is the source of energy used to drive biochemical activities. The three phosphoryl groups are labeled as alpha (α), beta (β), and, for the terminal phosphate, gamma (γ). The triphosphate group is joined to ribose via a phosphoester linkage. The three phosphoryl groups are joined by two phosphoanhydride linkages. Biochemists refer to the phosphoanhydride linkages as high-energy bonds. When these bonds are broken, the released chemical energy powers the energy-requiring processes within the cell.
When the bond between the gamma and beta phosphoryl groups is broken, the compound ADP forms and releases a phosphate molecule. When the linkage between the beta and alpha phosphoryl groups is broken, the compounds AMP and pyrophosphate form (see figure 2). The phosphoanhydride linkage joining the two phosphate moieties of pyrophosphate can be further broken down into two phosphate molecules, releasing additional energy that can also be used to power cellular activities.
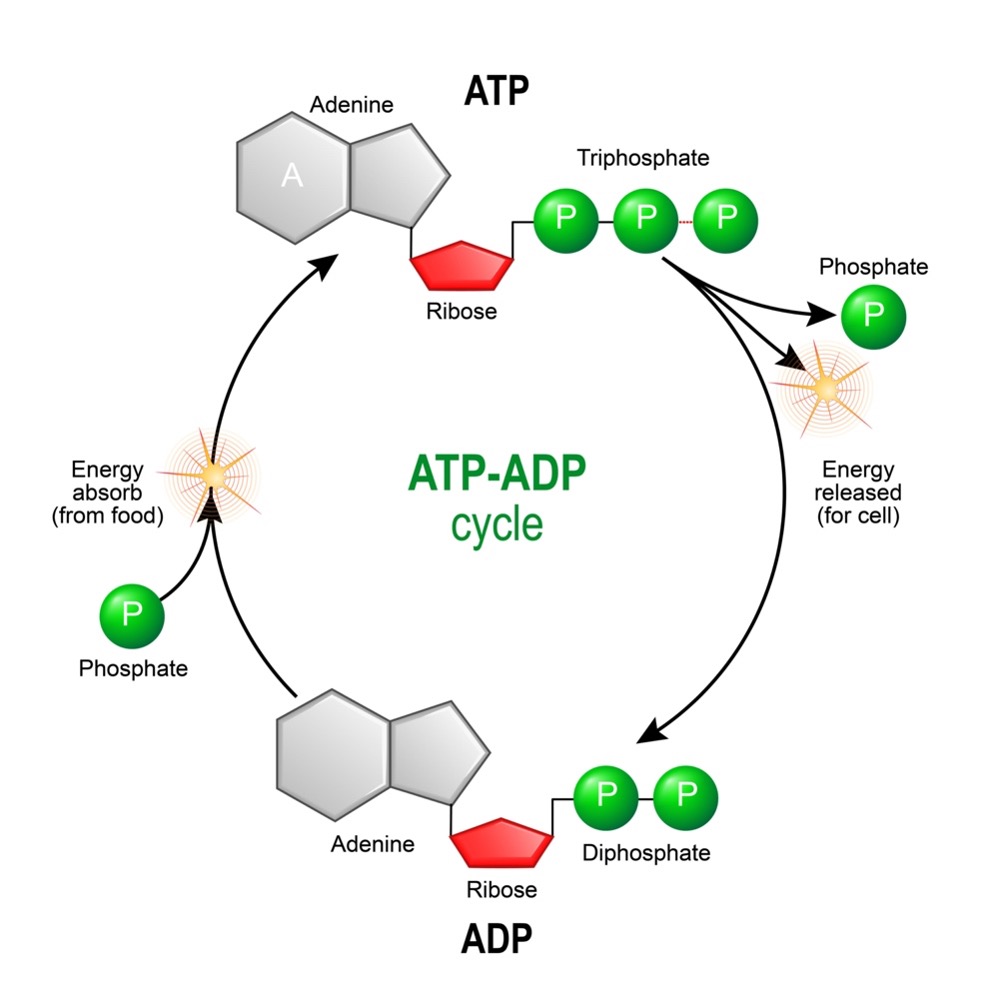
Figure 2: Hydrolysis of ATP
Credit: Shutterstock
ATP forms in the cell through a variety of biochemical pathways. These pathways involve the breakdown of “fuel” molecules (such as sugars and fats) which, in turn, release chemical energy that drives the formation of ATP from ADP and inorganic phosphate. Ultimately, most of the cell’s ATP is made by the enzyme complex, ATP synthase.
The Cellular Economy
The use of a single molecular species (for the most part) to store and supply energy for biochemical operations relies on the same logic that undergirds the use of currency to drive an economy. Hence, ATP is called the energy currency of the cell.
To appreciate the ingenuity of using a single molecular species as the energy currency, imagine that you work for a farmer. Without currency, the only way he can pay you for your labor is with goods, say oranges produced in his orchard. If you need a pair of shoes, you must hope that the shoemaker needs oranges. If he doesn’t, you can’t get the shoes. You now need to find someone who will take the oranges and provide you with goods that the shoemaker needs.
If no one wants oranges, you are out of luck procuring any goods and services. And you (and anybody else for that matter) are unlikely to work for the farmer unless he agrees to give you some other commodity for your labor that you can easily exchange for the goods and services you desire. If he can’t, then you will lose a job and he will lose labor.
Currency eliminates all these problems. Because it is a standardized medium of exchange that has value, it can be used to pay laborers who, in turn, can use it to buy whatever products they want. And the shopkeepers can accept currency from them because they have the confidence that they can use it to buy whatever they would like.
In the same vein as this imaginary scenario, the cell could employ a type of bartering system. Instead of using ATP as an energy store and currency, the energy released when the chemical bonds of “fuel” molecules break apart, in principle, could be directly used to drive biochemical activities. However, if specific biochemical operations were coupled to the breakdown of specific molecules (or the breakdown of specific bonds within specific molecules), it would lead to molecular-scale inefficiencies in the same way that bartering frustrates economic efficiency. Specific biochemical processes in the cell could only take place if the just-right type of “fuel” molecule was present in the cell or if it was at the just-right levels. For example, if a certain biochemical process required the breakdown of a specific chemical bond in the sugar molecule, glucose, and if insufficient levels of glucose existed in the cell, the necessary biochemical process couldn’t take place until other metabolites were converted into glucose.
This conversion most likely would involve a highly convoluted series of chemical reactions—in the same way that multiple exchanges must take place in a barter economy to satisfy everyone involved. In fact, the inefficiency of a barter-based metabolic economy would be so extreme that it raises questions—at least for me as a biochemist—about the possibility of life without some type of energy currency.
Additionally, ATP’s use as an energy currency helps maintain balanced biochemical processes in the cell. In the same way that economies can be slowed down or ramped up by controlling the amount of currency, the metabolic operations in the cell can be dialed up or down based on ATP levels. When ATP levels are high, energy-harvesting pathways slow down and energy-consuming pathways speed up. When ATP levels are low, the reverse scenario takes place with energy-generating pathways speeding up their operation and energy-consuming pathways slowing down.
Biochemical Energy Currency and the Watchmaker Argument
The remarkable similarity between the role ATP plays in the cell’s economy and currency plays in human economies allows us to advance a version of the Watchmaker argument for God’s existence and role in the origin and design of life.
British natural theologian William Paley (1743–1805) proposed this argument by highlighting the characteristics of a watch, specifically the complex interaction of its precision parts for the purpose of telling time. He maintained that the contrivances integral to the watch’s design implied the work of an intelligent designer—a watchmaker. Paley demonstrated that systems in biology share the same characteristics that cause us to recognize a watch as the product of a designer. Therefore, by analogy, as a watch requires a watchmaker, so too, life requires a Creator.
In my book The Cell’s Design, I argue that the latest insights into the structure and function of biomolecules add new vitality to Paley’s Watchmaker argument. For example, there are many kinds of molecular-level biomachines that stand as strict analogs to human-made machines with respect to their architecture, operation, and assembly. As a case in point, some protein complexes, such as ATP synthase (the enzyme complex that makes ATP from ADP and phosphate), bear an astonishing similarity to motors designed by humans. These protein complexes come with rotors, stators, drive shafts, cams, turbines, and universal joints. The one-to-one relationship between the parts of human-made machines and the molecular components of biomachines is startling and further justifies Paley’s conclusion that life stems from the work of a Creator. We can add the discovery of ATP’s role as the cell’s energy currency to the revitalized Watchmaker argument.
The use of currency to generate economic efficiency is a human invention. In fact, I would submit that it might be the greatest, most ingenious human invention of all time—even greater than the wheel. If not for the invention of currency, efficient economies would be impossible—and so would human civilization. And if the use of currency to generate economic efficiency is an ingenious human design, then the use of biochemical energy currency must, too, reflect the ingenuity of an intelligent agent.
Why ATP?
Scientists now recognize the necessary role of a biochemical energy currency. But why was ATP “selected” as the energy currency? Why not some other biomolecule?
ATP serves universally as the biochemical energy currency. Every organism on Earth uses ATP to power its metabolic operations. From an evolutionary perspective, this recognition means ATP must have been employed as the cell’s energy currency in LUCA (the last universal common ancestor) and perhaps in the cells that preceded LUCA. In an evolutionary framework, ATP’s usefulness as the biochemical energy currency must have been an early invention.
Many evolutionary biologists would argue that ATP’s choice as the cell’s energy currency was happenstance. It resulted from the outworking of an unguided, undirected, evolutionary process that was constrained by history. In other words, there was no rhyme or reason for why ATP was selected as the cell’s energy currency. It’s just the accidental outcome of evolutionary history. Alternatively, other researchers propose that the forces of natural selection led to ATP as the optimal (or near optimal) choice as the cell’s energy currency.
As it turns out, biochemists have identified several reasons why ATP is well-suited for its role as the biochemical currency for the cell’s energy. This fact explains ATP’s ascendency to its status as the universal energy currency.
One reason relates to other roles ATP plays in the cell. Not only does ATP function as the cell’s energy currency, but, along with the ribonucleotides GTP, CTP, and UTP, ATP is one of the building block components for RNA. This class of biomolecules plays a critical role in expressing the information found in DNA. For example, the cell’s machinery produces messenger RNA by copying the information harbored in the DNA molecule in regions (called genes). The RNA copies, in turn, direct the production of specific proteins. These biomolecules then form the structures that comprise the cell’s components and carry out the cell’s operations. By utilizing one of the building blocks of RNA as the biochemical energy currency, gene expression is linked to the energy status of the cell. When the cell’s energy status is low, it’s best to minimize gene expression overall. On the other hand, it makes sense to accelerate the expression of genes that code for proteins that play a role in energy-harvesting processes.
Second, using ATP as both the cell’s energy currency and a building block component for RNA leads to metabolic simplicity. Instead of having a separate metabolic pool for the cell’s energy currency and, in this case, the building blocks needed for the biosynthesis of RNA, the cell can rely on a single pool of biomolecules that serves both biochemical operations.
A third reason why ATP was chosen instead of GTP, CDP, or UTP concerns how it interacts with proteins. Both CTP and UTP contain a pyrimidine as the nucleobase instead of a purine. Because purines consist of two fused molecular rings, they are more hydrophobic (lacking affinity for water) than pyrimidines, which consist of a single six-membered nucleobase. The increased hydrophobicity of purine nucleobases causes ATP and GTP, as a rule of thumb, to interact more strongly with proteins than CTP or UTP, an important quality for a molecule that functions as the cell’s energy currency.
For a fourth reason, we might ask why ATP instead of GTP? Guanine (GTP’s nitrogenous base) is made of carbon, nitrogen, and oxygen atoms. On the other hand, adenine (ATP’s nitrogenous base) lacks oxygen atoms in its structure. This compositional difference means that it takes fewer biosynthetic steps to make ATP compared to GTP. As a result, using ATP as the cell’s energy currency leads to more efficient utilization of the cell’s energy resources. In other words, it makes more sense to use a molecule that is less expensive to produce than one that is more expensive to produce as the cell’s energy currency.
In sum, there appears to be an exquisite rationale for the selection of ATP as the cell’s energy currency. This insight means that ATP’s designation as the biochemical energy currency wasn’t a frozen accident of a historically contingent evolutionary process. The recognition of a molecular logic in ATP as life’s molecular energy currency is consistent with two radically different views: ATP as the cell’s energy currency came about either by (1) an evolutionary adaptation driven by natural selection, or (2) the intentional design of a Creator.
Can Evolutionary Processes Generate a Cellular Currency System?
I contend that the intentional choice by a Creator is the best explanation for the selection of ATP as the biochemical energy currency. I question whether gradual, stepwise evolutionary processes could have produced an energy currency system. To understand why, let’s return to the analogy of human economies. For a currency to be accepted as a universal store of economic value, it must be accepted by virtually every actor in the economy—in effect, all at once. Those people who refuse to use the currency can’t fully take part in the economy, other than by bartering. They can’t truly be integrated into the economy and would participate only from the sidelines.
The same requirements would be true for the ATP-based cellular economy. For a cell to use ATP to power its metabolic reactions, all the biochemical processes must use ATP as an energy source all at once. Those biochemical reactions that don’t use ATP can’t fully participate in the cellular economy. These processes may still occur, but they would operate in a haphazard, unpredictable way that prevents them from reliably contributing to cellular metabolism. The failure of these sidelined metabolic activities to consistently contribute to the cell’s economy could be deleterious to the cell, particularly if they’re part of core biochemical operations.
In fact, the use of ATP as the energy currency molecule could not have emerged in a stepwise manner with a few reactions relying on ATP and the rest bartering. The inefficiency of bartering reactions would so overwhelm the cell’s operations that life would be impossible. Despite this problem, evolutionary biologists and origin-of-life researchers still seek an evolutionary explanation for the origin of ATP as the biochemical energy monetary system.
Insight from Prebiotic Chemistry
A team headed by biochemist Nick Lane from University College London took a different approach to the “why” question about ATP’s selection as the cell’s energy currency. Assuming an evolutionary origin, they wondered if chemical—instead of evolutionary—processes on early Earth could offer insight into the origin of ATP’s role as the cell’s energy currency.1
In an earlier study, this team showed that acetyl phosphate—a phosphorylating agent—could form under mild hydrothermal conditions from the reaction of thioacetate (which would reasonably form at hydrothermal events on early Earth from hydrogen sulfide and acetic acid) and inorganic phosphate. Acetyl phosphate is modestly stable and can survive for several hours, depending on pH and temperature. In other words, this compound has the just-right balance of chemical stability and reactivity. Acetyl phosphate can also transfer a phosphate group to: (1) ribose to make ribose-5-phosphate, (2) adenosine to make AMP, and (3) ADP to make ATP.2
The team reasoned that the transfer of the phosphate from acetyl phosphate to ADP may be a preferred reaction that explains ATP’s emergence as the universal biochemical currency. Their reasoning was justified. They showed that in the presence of ferric iron, acetyl phosphate transferred a phosphate group to ADP. The team discovered that no other phosphorylating agent successfully added a phosphate group to ADP. They also evaluated other ions as catalysts and discovered that none of them promoted the phosphorylation of ADP. Finally, they found that acetyl phosphate and ferric iron would not phosphorylate GTP, CDP, and UDP. The researchers discovered that this remarkable specificity stems from just-right, precise chemical interactions that uniquely exist for acetyl phosphate, ADP, and ferric iron.
This discovery carries profound implications. It means that the use of ATP as the cell’s energy currency isn’t a frozen accident that arises out of the constraints of a contingent evolutionary history. Nor is it due to natural selection. Instead, it’s due to a highly unusual chemical reaction. In other words, the use of ATP as the cell’s energy currency—from the perspective of prebiotic chemistry—has been prescribed by the laws of nature.
The research team’s responses are compelling:
“The emergence of ATP as the universal energy currency of the cell was not the result of a ‘frozen accident,’ but arose from the unique interactions of ADP and AcP.”3
“This implies that ATP could have become the universal energy currency of life not as the end-point of genetic selection or as a frozen accident, but for fundamental chemical reasons.”4
“Our results suggest that ATP became established as the universal energy currency in a prebiotic, monomeric world, on the basis of its unusual chemistry in water.”5
“It was very surprising to discover the reaction is so selective—in the metal ion, phosphate donor, and substrate—with molecules that life still uses.”6
As interesting as this finding may be, the chemistry the research team discovered lacks geochemical relevance. There are a few reasons to be skeptical that this chemical process could take place on early Earth.
- It’s questionable that geochemically relevant prebiotic reactions could generate ADP.
- It’s questionable that a source of inorganic phosphate would be available.
- It’s questionable that ferric iron would be abundant on early Earth.
Evidence of Intentional Design
Despite these doubts, let’s assume that this reaction did take place on early Earth as part of the process of chemical evolution.
If we do, the evidence for teleology (design, purpose) is still undeniable. It is eerie that a highly unusual, just-right chemical process uniquely produces ATP—a molecule with a just-right set of properties that’s ideally suited to serve as the cell’s energy currency—under presumed prebiotic conditions. Neither accident nor natural selection is responsible for ATP’s origin. This biomolecule appears to be prescribed by the laws of nature. Its existence appears to be intentional. These coincidences highlight the design and purpose of ATP as the biochemical energy currency.
When we combine these remarkable coincidences, the Watchmaker analogy (described above), and the challenge of imagining how ATP could have emerged as the energy currency for the cell through evolutionary processes, we are left with only one reasonable conclusion: Whether one adopts a creation model or evolutionary approach to life’s genesis, ATP’s origin, properties, and biochemical role point to the necessary action that a Creator must have played in the origin and design of life.
And that conclusion is money in the bank.
Resources
- Fit for a Purpose: Does the Anthropic Principle Include Biochemistry? by Fazale Rana (book)
- The Cell’s Design: How Chemistry Reveals the Creator’s Artistry by Fazale Rana (book)
Biochemical Anthropic Principle
- “DNA’s Fine-Tuned Structure Minimizes Harmful Tautomers” by Fazale Rana (article)
- “Just-Right RNA Structure Points to Intentional Design” by Fazale Rana (article)
- “The Logic of DNA Replication Makes a Case for Intelligent Design” by Fazale Rana (article)
- “How the Central Dogma of Molecular Biology Points to Design” by Fazale Rana (article)
- “Is the Optimal Set of Protein Amino Acids Purposed by a Mind?” by Fazale Rana (article)
- “A Periodic Table for Protein Structures Reveals Biochemical Design” by Fazale Rana (article)
- “Evidence That the Cell’s Metabolism Is Planned” by Fazale Rana (article)
- “Krebs Cycle Origin Brings Case for Creation Full Circle” by Fazale Rana (article)
- “Molecular Logic of the Electron Transport Chain Supports Creation” by Fazale Rana (article)
The Watchmaker Argument
- “ATP Synthase Ratchets Up the Case for Intelligent Design” by Fazale Rana (article)
- “Addressing the Concerns of a Critic and the Case for Intelligent Design” by Fazale Rana (article)
Endnotes
- Silvana Pinna et al., “A Prebiotic Basis for ATP as the Universal Energy Currency, PLoS Biology 20 (October 4, 2022): e3001437, doi:10.1371/journal.pbio.3001437.
- Alexandra Whicher et al., “Acetyl Phosphate as a Primordial Energy Currency at the Origin of Life,” Origins of Life and Evolution of Biospheres 48 (March 3, 2018): 159–79, doi:10.1007/s11084-018-9555-8.
- PLOS, “Ancient Chemistry May Explain Why Living Things Use ATP as the Universal Energy Currency,” ScienceDaily (October 4, 2022).
- Pinna et al., “A Prebiotic Basis for ATP.”
- Pinna et al., “A Prebiotic Basis for ATP.”
- PLOS, “Ancient Chemistry May Explain.”