DNA’s Fine-Tuned Structure Minimizes Harmful Tautomers
I thought I had found the perfect Valentine’s Day gift for Amy. We were dating at the time and I bought her a Gizmo stuffed animal. We had seen the horror comedy Gremlins the previous summer (1984) and both enjoyed it. Gizmo, the movie’s main character, is a cute and adorable version of a mogwai (small, fictional creature). When I saw Gizmo on the store shelf, I couldn’t resist. I thought Amy would really like to have her own mogwai. For the record, Amy wasn’t that impressed with my gift. In retrospect, I probably should have given her a nice box of chocolates.
Even though Amy wasn’t all that enthusiastic about having her own mogwai, it doesn’t change the enthusiasm that horror movie fans have for Gremlins. The movie’s plot begins to unfold when teenager Billy Peltzer receives a mogwai as a gift from his father. He had purchased the cute, furry, and sweet mogwai from an antique store in Chinatown. But Billy’s father was warned:
- Do not expose the mogwai to light, because it will kill it.
- Do not let it contact water.
- Do not feed it after midnight.
As expected, one of Billy’s friends accidentally spills water on Gizmo and the creature spawns several more mogwai. These mogwai are much more mischievous than Gizmo and they trick Billy into feeding them after midnight. After they feed, they form cocoons and later emerge as reptilian creatures called gremlins. These newly spawned mogwai are led by the villainous Stripe, who escapes from the Peltzers’ home. Stripe jumps into the pool at the local YMCA, producing numerous gremlins who wreak havoc on the town.
This catastrophe all came about because, unbeknownst to Billy’s father, mogwai exist in two forms, one cute and adorable and the other ugly and evil.
DNA’s Nucleobases Can Be Good or Bad
Like mogwai, the nucleobases that contribute to the structure of DNA can also take on two forms, called tautomers. Biochemists are fully aware that one tautomer is beneficial, contributing to DNA’s structural integrity. The other form is harmful, triggering mutations in DNA during the replication process.
A researcher from Trinity College in Dublin, Ireland, studied tautomerism in the nucleobases of DNA and discovered it could actually be worse.1 As it turns out, the nucleobases’ composition in DNA is ideal. If DNA had been constructed with other nucleobases (conceivably available on early Earth), mutations caused by nucleobase tautomerism would have been more frequent, perhaps making life impossible.
This insight about tautomerism in the DNA nucleobases adds to scientists’ fundamental understanding of the structure of DNA. It answers an important “why” question; namely, why were adenine, guanine, and cytosine selected specifically to form DNA’s structure? The answer also has philosophical and theological implications. DNA’s structure displays a type of biochemical fine-tuning that reflects an exquisite molecular logic. Both indicate that DNA’s structure must be the work of an intelligent Agent.
Before I unpack the researcher’s work and delve into the teleological implications, a brief primer on DNA’s structure will be helpful. If you are familiar with the structure of DNA, feel free to skip ahead to Tautomers.
DNA’s Structure
DNA consists of two chain-like molecules (polynucleotides) that align and twist to form the DNA double helix. The cell’s machinery generates polynucleotide chains by linking together four different subunit molecules called nucleotides. DNA is built from the nucleotides adenosine, guanosine, cytidine, and thymine, famously abbreviated A, G, C, and T, respectively. The nucleotide molecules that make up the strands of DNA are complex molecules (see figure 1), consisting of both a phosphate moiety, and a nucleobase (either adenine, guanine, cytosine, or thymine) joined to a 5-carbon sugar (deoxyribose).
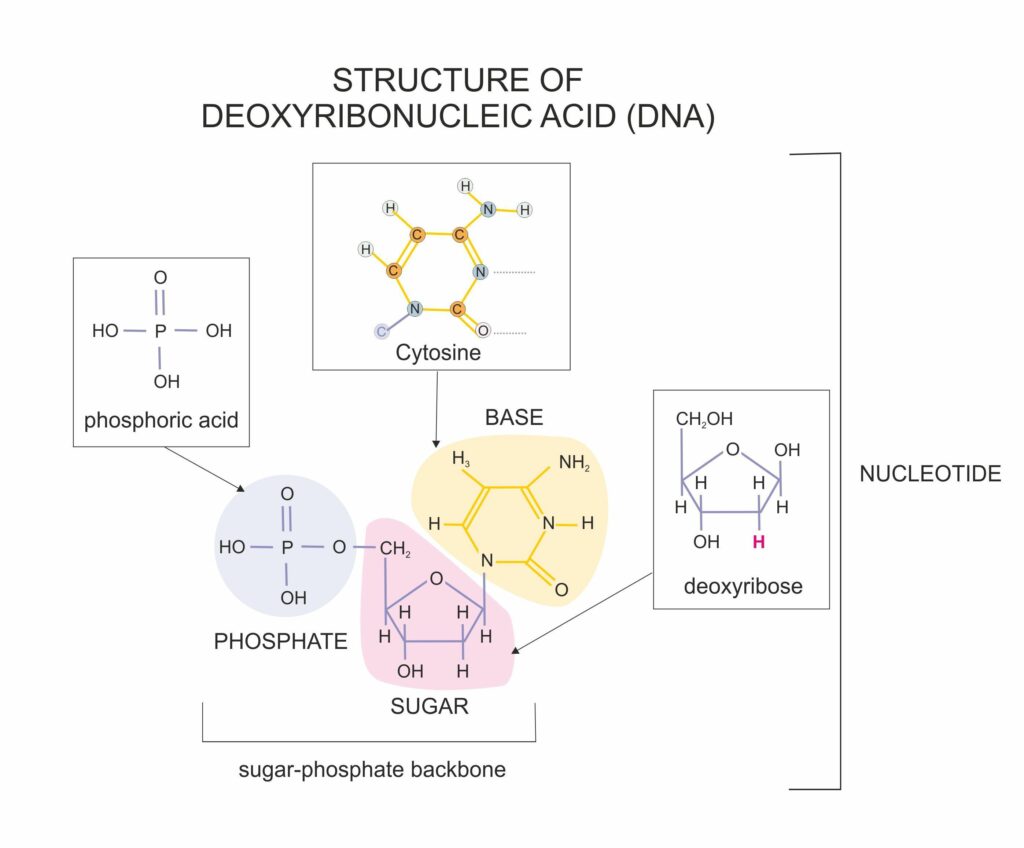
Figure 1: A Nucleotide
Credit: Shutterstock
The backbone of the DNA strand consists of alternating phosphate and deoxyribose moieties. This alternating back arises when the cell’s machinery repeatedly links the phosphate group of one nucleotide to the deoxyribose unit of another nucleotide. The nucleobases extend as side chains from the backbone of the DNA molecule and serve as interaction points (like ladder rungs) when the two DNA strands align and twist to form the double helix (figure 2).
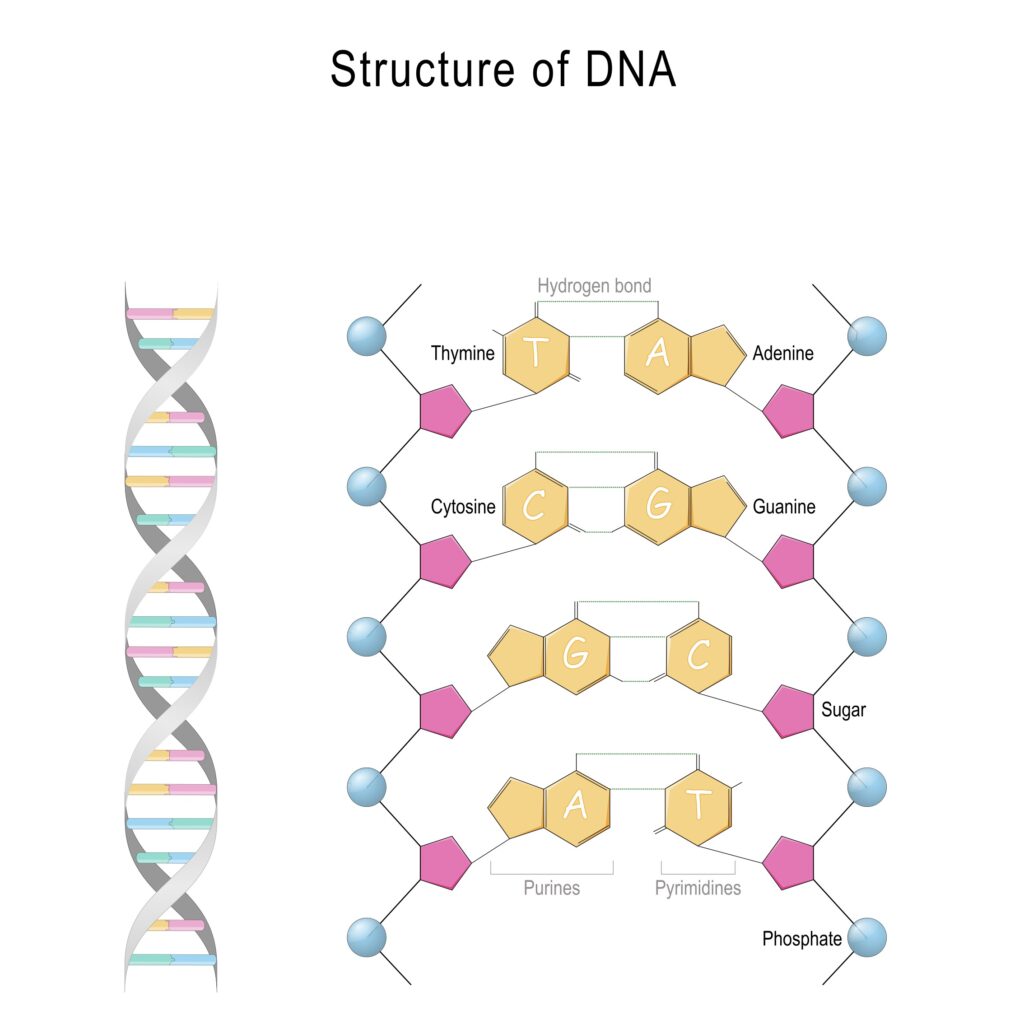
Figure 2: The Structure of DNA
Credit: Shutterstock
When the two DNA strands align, the adenine (A) side chains of one strand always pair with thymine (T) side chains from the other strand. Likewise, the guanine (G) side chains from one DNA strand always pair with cytosine (C) side chains from the other strand.
When the side chains pair, they form crossbridges between the two DNA strands. The length of the A-T and G–C cross bridges is nearly identical. Adenine and guanine are both composed of two rings and thymine (uracil) and cytosine are composed of one ring. Each crossbridge consists of three rings.
When A pairs with T, two hydrogen bonds mediate the interaction between these two nucleobases. Three hydrogen bonds accommodate the interaction between G and C. The specificity of the hydrogen bonding interactions accounts for the A-T and G-C base-pairing rules (figure 3).
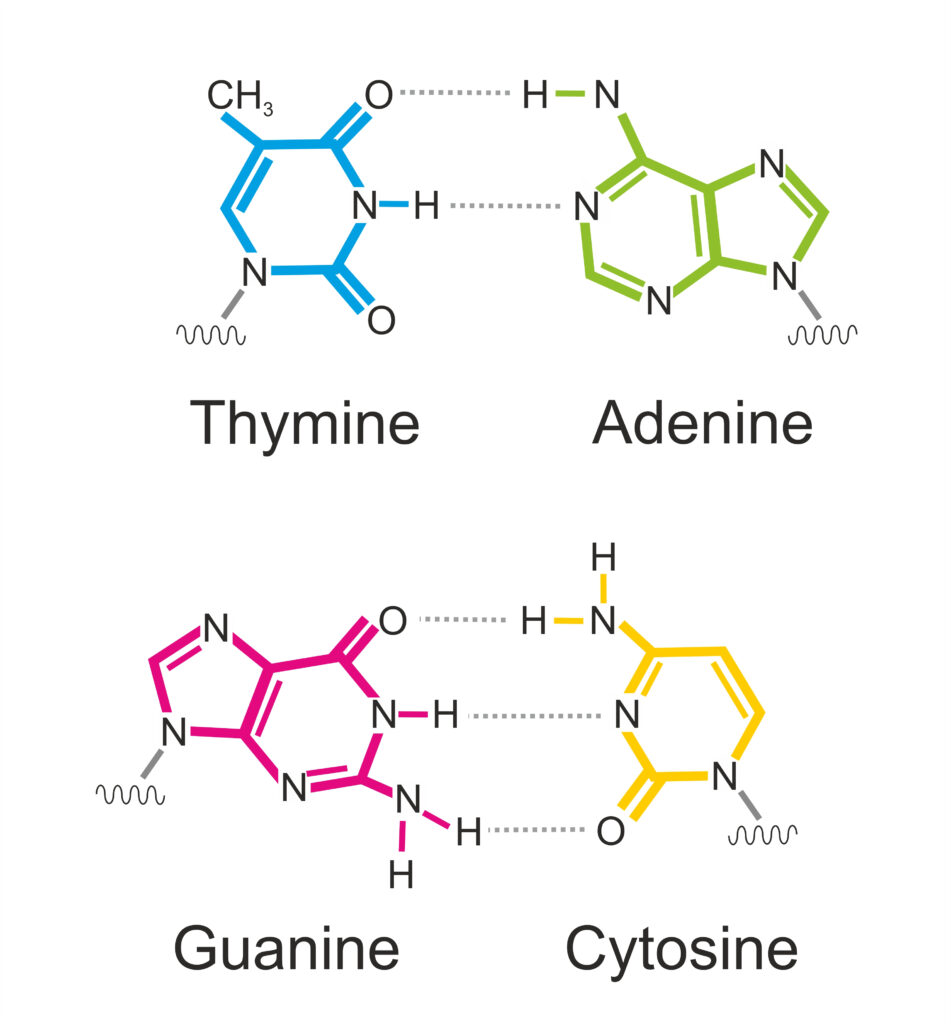
Figure 3: Base Pairing in DNA
Credit: Shutterstock
Tautomerism
Tautomers are organic compound pairs that mutually interconvert one into another through a special type of chemical reaction called tautomerization. The reaction involves a switch between adjacent single and double bonds accompanied by the formal migration of a hydrogen atom from an atom that takes part in the original single bond to an atom that takes part in the new single bond (which results from the single bond-double bond switch).
The most widely known example of tautomerism is called keto-enol tautomerization (figure 4).
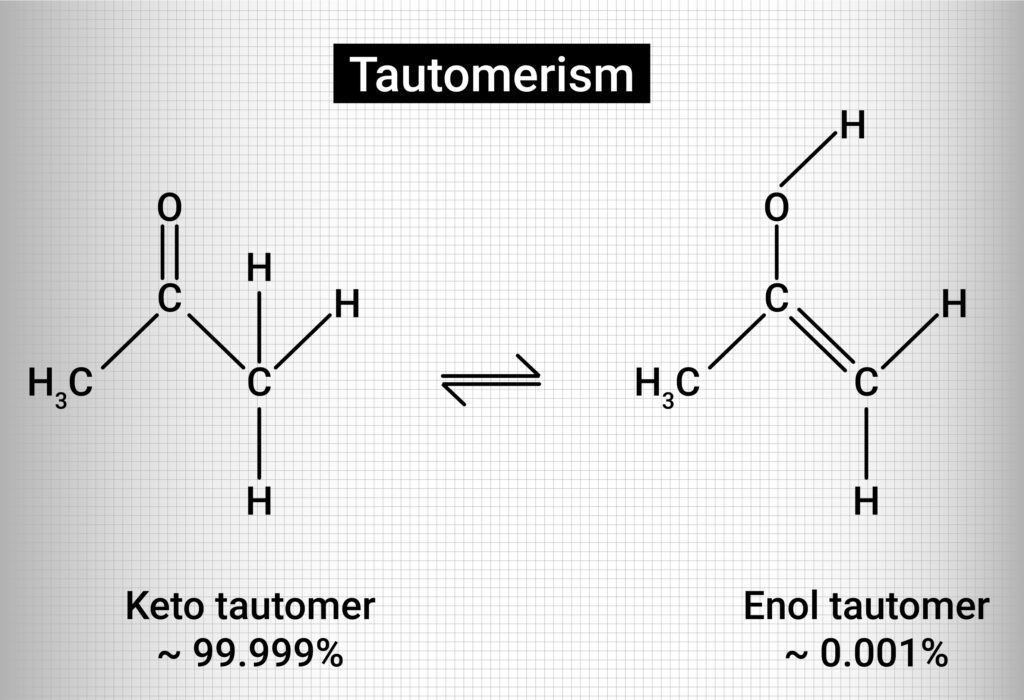
Figure 4: Keto-Enol Tautomerization
Credit: Shutterstock
If a compound is capable of tautomerization, it will spontaneously generate both tautomers (keto and enol) when dissolved in solution, if the solution conditions are right. For keto-enol tautomers, the keto form has much greater chemical stability and is the thermodynamically favored form. For this reason, in solution, the keto form will exist at a much higher concentration than the enol form. In addition to keto-enol tautomers, other tautomers exist such as the amide-imide tautomers, amine-imine tautomers and the lactam-lactim tautomers (figure 5).
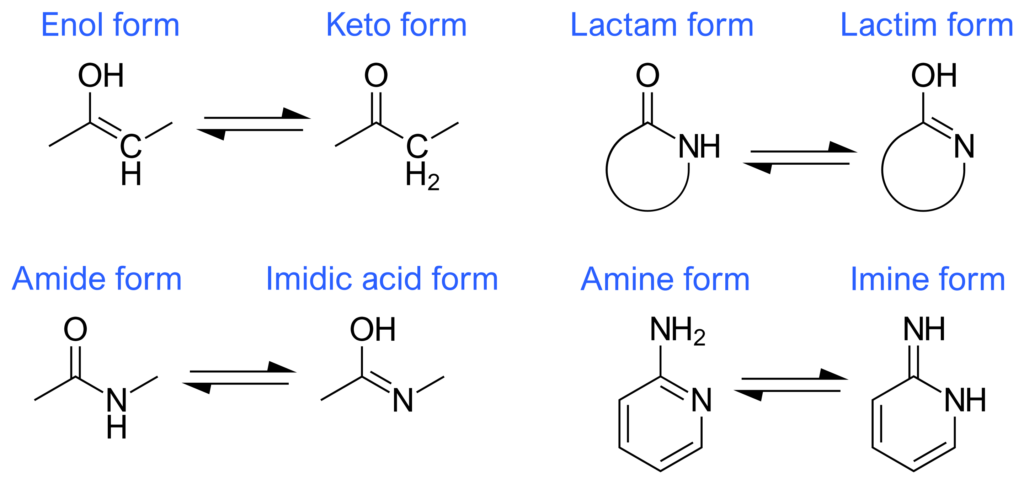
Figure 5: The Most Common Types of Tautomers
Credit: Wikipedia
Tautomerism and DNA
The nucleobases found in DNA all undergo tautomerization reactions in solution.
Adenine and cytosine both interconvert between amino and imino forms (with the amino form analogous to the keto form and the imino form analogous to the enol form). The amino form predominates in both adenine and cytosine because it’s the more thermodynamically stable tautomer.
Guanine and thymine both interconvert between lactam and lactim forms (with the lactam form analogous to the keto form and the lactim form analogous to the enol form). The lactam form predominates in both guanine and thymine because it’s the more thermodynamically stable tautomer.
The consequences of nucleobase tautomerization aren’t good. The hydrogen bonding interactions of the imino forms of adenine and cytosine and the lactim forms of guanine and thymine differ from the amino and lactam forms, respectively. These differences cause mispairing between the nucleobases of the two DNA strands, which can cause mutations during DNA replication.
DNA’s chief function is storing the information needed to build the cell’s components and the cellular machines that carry out vital processes within the cell. But tautomerism of DNA’s nucleobases poses a threat to the integrity of the information housed in the nucleotide sequences of DNA because it causes mispairing that can lead to mutations, which compromise the integrity of the information stored in DNA.
DNA Is Optimized to Minimize Tautomerism’s Harmful Effects
Because of the importance of nucleobases, D. A. Mac Dónaill from Trinity College studied the tautomerism in DNA with an eye toward a larger question: Why were the four nucleobases adenine, guanine, cytosine, and thymine selected for DNA?
As Mac Dónaill points out, DNA would have been better suited to be built from more than four nucleobases (and, hence, four nucleotide subunits). A larger set of nucleotide subunits would allow the information in DNA which specifies the complex functionality of the cell’s machinery to be stored in shorter sequences.
So, did nature just simply stumble onto these four nucleobases as a consequence of an unguided, historically contingent evolutionary process? Was it prebiotic availability that influenced their selection? Or were there other reasons?
To answer these questions, Mac Dónaill performed calculations using quantum mechanics to compare the stability of the tautomers of the nucleobases that naturally occur in DNA with the stability of tautomers of nonnatural nucleobases, such as isoguanine, isocytosine, and xanthine. He learned that the “keto” forms of adenine, guanine, cytosine, and thymine displayed greater stability (relative to the “enol” forms) compared to the tautomers of the nonnatural nucleobases he studied. The greater stability of the “keto” form of the nucleobases in DNA significantly minimizes the mispairing of nucleobases. If other nucleobases were selected for DNA, it would have been worse. This means that the nucleobases that make up DNA form what appears to be a uniquely optimal set that ensures the stability of DNA’s information content by minimizing base mispairing that occurs because of tautomerism.
Other work (which I describe in The Cell’s Design and Fit for a Purpose) indicates that the four nucleobases of DNA have other unique properties. For example, the adenine-thymine and guanine-cytosine pairs are the only ones that cause a distortion in the DNA double helix if misfiring of the bases occurs.
The four nucleobases also happen to be unique with respect to their photostability. Adenine, guanine, cytosine, and thymine withstand the damaging effects of UV radiation better than nonnatural nucleobases. In other words, the nucleobases found in DNA display multidimensional optimality, all of which ensure the integrity of the information housed within the DNA molecule’s structure.
DNA and the Case for Intentional Design
In both The Cell’s Design and Fit for a Purpose, I describe how all other aspects of DNA’s compositional makeup and structural features are optimized for DNA’s role as an information storage molecular system, including the nucleobase composition and structure. This insight from Mac Dónaill adds to the features of DNA’s structure that are optimized.
For an engineer, optimization is synonymous with design. And optimized engineered systems don’t just happen—they result from engineers carefully fine-tuning their designs. Optimization requires forethought, planning, and careful attention to detail. DNA’s optimized structure constitutes evidence for the intelligent design of DNA.
DNA’s Structure and Anthropic Coincidences
The case for intentional design goes beyond the optimized structure of DNA. The fine-tuning of DNA’s structure hints at a much deeper and richer teleology.
The rationale and molecular logic that undergird the selection of the four nucleobases found in DNA make it unlikely that unguided, directionless, historically contingent evolutionary processes just stumbled upon the four nucleobases found in DNA. If this is the explanation for DNA’s structure we would expect the nucleobase composition of DNA to have little rhyme or reason to it.
It’s also unlikely that natural selection is exclusively responsible for the choice of nucleobases found in DNA. This explanation would be plausible if the nucleobases in DNA were optimized for a single feature. But the nucleobases in DNA are simultaneously optimized with respect to at least three features. It’s remarkable—and a bit eerie—that the adenine-thymine and guanine-cytosine pairs appear to uniquely possess: (1) the just-right tautomeric stability, (2) the just-right photostability, and (3) the just-right tendency to distort the DNA double helix when mispairing of the nucleobases occurs. What are the odds? The simultaneity of these three just-right features for the nucleobases in DNA can’t merely be a coincidence. There must be something more.
I would assert that the just-right properties of adenine, guanine, cytosine, and thymine that are critical to life point to a biochemical anthropic principle. That is, they appear to be prearranged by the laws of nature. The nucleobase composition of DNA appears to be prescribed by the laws of nature. These laws constrain the nucleobase composition (and the rest of DNA’s structure). These constraints also happen to yield a set of nucleobases with the precise properties needed to ensure DNA is optimally suited for its role as an information storage molecule—a critical property if life is to exist. And, as it turns out, this set of nucleobases appears to be uniquely suited for DNA.
That is to say, DNA appears to be uniquely fit for a purpose. And fitness for purpose is a hallmark of intentional design.
Thanks to its just-right nucleobase composition, DNA is less demanding than a mogwai. It can tolerate the light. It can get wet. And it will still have the structural integrity to spawn copies of itself. All by design.
Resources for Further Exploration
- The Cell’s Design: How Chemistry Reveals the Creator’s Artistry by Fazale Rana (book)
- Fit for a Purpose: Does the Anthropic Principle Include Biochemistry? by Fazale Rana (book)
The Optimal Structure of DNA
- “DNA: Designed for Flexibility” by Fazale Rana (article)
- “DNA Soaks Up Sun’s Rays” by Fazale Rana (article)
DNA and the Anthropic Principle
- “The Logic of DNA Replication Makes a Case for Intelligent Design” by Fazale Rana (article)
- “How the Central Dogma of Molecular Biology Points to Design” by Fazale Rana (article)
Endnotes
- D. A. Mac Dónaill, “Tautomerism as a Constraint on the Composition of Alternative Nucleotide Alphabets,” Proceedings of the Eighth International Conference on Artificial Life, ACM Digital Library (December 9, 2002): 106–110.






