ATP Synthase Ratchets Up the Case for Intelligent Design
I love reading Shakespeare’s plays and seeing them performed. One of the things that fascinates me about the Bard’s work is his use of dramatic devices such as a “play within a play.” He effectively employed this technique in both A Midsummer Night’s Dream and Hamlet as a way to advance the plot, with each mock performance serving as a mirror for the events and the themes of the main play.
Researchers recently discovered the biochemical equivalent of a play within a play—or more appropriately a motor within a motor motif—for the enzyme F0-F1 ATP synthase.1 This discovery effectively provides important insight into the way this enzyme functions. It also helps to advance the case for intelligent design.
F0-F1 ATP Synthase
The recognition that many protein complexes function as molecular-level machines is one of the most remarkable advances in biochemistry during the last part of the twentieth century.
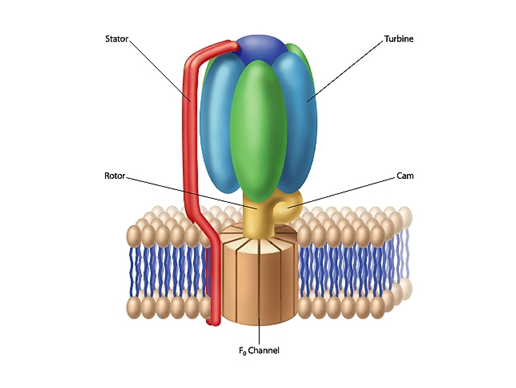
One such system is the protein complex F0-F1 ATP synthase. As I point out in The Cell’s Design, this rotary motor, found throughout nature, plays a central role in harvesting energy for cellular use. F0-F1 ATP synthase associates with cell membranes. The F1 portion of the complex is mushroom-shaped and extends above the membrane’s surface. The “button” of the mushroom corresponds literally to an engine turbine. The F0-F1 ATP synthase turbine interacts with the complex’s rotor (the “stalk” of a mushroom). The flow of positively charged hydrogen ions (or in some instances sodium ions) through the F0 component, embedded in the cell membrane, drives the rotation of the rotor. A rod-shaped protein structure, extending above the membrane surface, serves as a stator. This protein rod interacts with the turbine, holding it stationary as the rotor rotates.
The electrical current, flowing through the channels of the F0 complex, is transformed into mechanical energy that drives the rotor’s movement. A cam, extending at a right angle from the rotor’s surface, causes displacements of the turbine. These back-and-forth motions are used to produce ATP (adenosine triphosphate). The cell uses this compound as a source of chemical energy to drive the operation of cellular processes.
The Motor Within
While biochemists generally understood the operation of this enzyme, they lacked a detailed understanding of the chemical mechanism that drives the generation of ATP from ADP (adenosine diphosphate) and Pi (an inorganic phosphate molecule)—until now. New work by scientists from Sweden reveals the missing mechanistic details.
It turns out that the chemical reaction that generates ATP from ADP and Pi occurs at three distinct sites located on the turbine portion of the F1 complex. Specifically, these sites reside in the three grooves between the subunits of the turbine. Each time the rotor turns 120° a molecule of ATP is made, with three molecules of this high-energy compound generated for one full rotation of the rotor.
The researchers discovered that the 120° step consists of two steps comprised of 90° and 30° rotations, respectively. These sub-steps correspond to two distinct transition states as ATP is made from ADP and Pi. Once these transition states are formed, two separate chemical barriers in the turbine groove force the reaction to ratchet forward, thereby preventing the reverse reaction. In other words, the reaction is driven to completion by a Brownian motor, a special type of molecular-scale machine.
The bottom line: there is a motor within a motor architecture for the F0-F1 ATP synthase. The rotary motor translates electrical energy into mechanical energy, thereby erecting the energy barrier for the Brownian motor to drive ATP production.
ATP Synthase and the Case for Intelligent Design
It is remarkable to note the similarity between the structure and general operation of ATP synthase and a man-made rotary motor. This similarity extends even to the Brownian motor located within the ATP synthase rotary motor, a molecular-scale machine that drives ATP production.
Brownian motors are based on the Brownian ratchet, a conceptual machine devised in 1912 by Polish physicist Marian Smoluchowski. Researchers are now exploring the use of Brownian ratchets in nanodevices as a way to power controlled motion at the molecular scale. It is provocative to think that this technology is an integral part of F0-F1 ATP synthase. Plus, as I note in The Cell’s Design, a number of other biochemical systems operate as Brownian motors as well.

The stark resemblance between man-made machines and molecular motors invigorates Paley’s Watchmaker argument and helps make the case that life stems from the work of a Creator. In the case of the F1-F0 ATP synthase, the Watchmaker argument based on the general operation of this biomotor is mirrored by the Brownian motor that operates within.
Endnotes
- Tamas Beke-Somfai, Per Lincoln, and Bengt Norden, “Double-Lock Ratchet Mechanism Revealing the Role of αSER-344 in F0F1 ATP Synthase,” Proceedings of the National Academy of Sciences, USA 108 (2011): 4828–33.



