Bacterial Flagellum Structure Stacks the Case for Intelligent Design
My mother’s side of the family is Germans from Russia. Drawn to Russian culture, my mother loved collecting babushka dolls. When she downsized a few years ago, we wound up inheriting her collection.

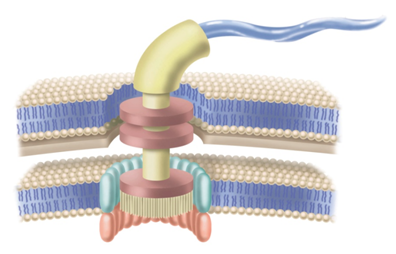
Scientists recently discovered the biochemical equivalent of Russian nesting dolls. They demonstrated that the protein complex that plays a role in assembling the bacterial flagellum’s outer structures is a structural and functional analog to the F1-F0 ATP synthase biomolecular motor.1 During the assembly process, the so-called export apparatus is housed within the flagellum—a rotary motor within a rotary motor.
This work not only fascinates biochemists (who may or may not be drawn to Russian culture), but also extends the case for intelligent design.
The Bacterial Flagellum
As discussed previously, over 40 different kinds of proteins make up the bacterial flagellum. These biomolecules function in concert as a literal rotary motor whose components include a rotor, stator, drive shaft, bushing, universal joint, and propeller. The bacterial flagellum is essentially a molecular-sized electrical motor (the flow of positively charged hydrogen ions through the motor proteins located in the bacterial inner membrane powers the flagellum’s rotation) directly analogous to man-made rotary motors.
The Manufacture of the Bacterial Flagellum
In my book The Cell’s Design and in last week’s article, I point out that production of the bacterial flagellum resembles a well-orchestrated manufacturing process. It ensures that the proper proteins are present at the proper time during assembly. (Take a look at the video below for a nice overview of flagellum assembly.)
Production begins with the assembly of the flagellum’s basal body component, consisting of the rotor and stator. The rod-like drive shaft, bushings, universal joint, and flagellar whip assemble sequentially from the basal body outward. To affect the assembly, proteins are brought to the basal body and then transported through its central channel to form the drive shaft. The bushings are assembled in a similar manner. But in addition to passing through the basal body’s central channel, the bushing proteins also pass through the hollow interior of the drive shaft, and so on and so forth until the entire flagellum is put together.
The export apparatus, a protein complex positioned below the basal body, controls the transport process. A team of bioscientists from Japan recently discovered that the architecture of the export apparatus is nearly identical to that of the F1-F0 ATP synthase.
F1-F0 ATP Synthase
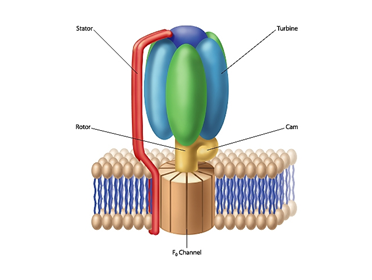
As I point out in The Cell’s Design and in a previous article, this rotary motor consists of two components: F1 and F0. The F1 portion of the complex extends above the cell membrane’s surface. Part of the F1 component literally corresponds to an engine turbine. The turbine interacts with the “rotor.” The flow of positively charged hydrogen ions (or in some instances, sodium ions) through the F0 component embedded in the cell membrane drives the rotation of the rotor. A rod-shaped protein structure also extends above the membrane surface and serves as a stator. This protein rod interacts with the turbine, holding it stationary as the rotor rotates.
Biomolecular Motors and the Case for Intelligent Design
In The Cell’s Design, I argue that the stark resemblance between man-made machines and molecular motors, like the bacterial flagellum and F1-F0 ATP synthase, reinvigorates Paley’s Watchmaker argument and, in doing so, helps make the case that life stems from the work of a Creator.

In light of this similarity, it is provocative to think that the export apparatus nested within the structure of the bacterial flagellum is yet another rotary motor, a system structurally and mechanistically similar to F1-F0 ATP synthase. (and it doesn’t stop there. As I discussed a few weeks ago, nested with the F1-F0 ATP synthase structure is a Brownian motor).
Why the Similarity?
The scientists who discovered the similarity between the export apparatus and F1-F0 ATP synthase speculated that the two motors must share a common evolutionary origin. The sole basis for this speculation is the structural and mechanistic similarity of the motors. If not for this similarity, there would be no reason to think that the export apparatus and the F1-F0 ATP synthase evolved from a common ancestral protein complex.
Yet, if life’s chemistry stems from the work of a Creator—as indicated by the structural and operational similarity between the molecular motors and man-made machines—it is not surprising that protein complexes like the export apparatus and the F1-F0 ATP synthase would share common design elements. It is not unusual for human designers to use the same designs over and over. In fact, it is a highly efficient way to create.
In other words, the common design of the export apparatus and the F1-F0 ATP synthase further adds to the stack of evidence for a Creator.
Endnotes
- Tatsuya Ibuki et al., “Common Architecture of the Flagellar Type III Protein Export Apparatus and F- and V-Type ATPases,” Nature Structural & Molecular Biology 18 (2011): 277–82.




