A Cornucopia of Evidence for Intelligent Design: DNA Packaging of the T4 Virus
Thanksgiving is my favorite holiday. I love the food, fellowship, and the chance to reflect on the abundance of blessings in my life. I’m filled with a spirit of gratitude.
I experienced this same feeling of thankfulness after thinking about recent scientific research from the Catholic University of America in Washington DC. These scientists have gained new insight into the structure and function of the DNA packaging machine of the T4 virus.1 Their discoveries have uncovered a cornucopia of new evidence for intelligent design.
The Infection Process
Viruses are infectious agents that consist of a protein capsule that houses genetic material (either DNA or RNA). Multiple copies of identical protein subunits interact to form the capsid. Some viruses also possess a protein tail that extends from the base of the viral capsid, which also consists of several protein subunits.
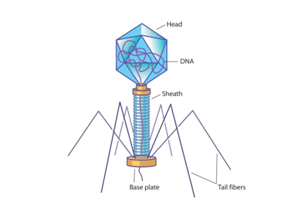
Viruses infect cells by binding to the surface of a target cell and injecting their own genetic material into the cell. When present, the viral tail binds the virus to the target cell’s surface and injects the viral genetic material into the host cell.
Once inside the cell, the viral genetic material uses the host cell’s enzymatic machinery to make copies of itself and its proteins, which then assemble to form multiple copies of the virus. With time, the newly produced virus particles cause the host cell to rupture, releasing the nascent viruses to repeat the infectious cycle.
The T4 virus infects the bacterium, E. coli. The embedded video clip depicts the binding of the T4 virus to the surface of E. coli followed by the injection of its genetic material (DNA) into the host cell.
The T4 Virus’ DNA Packaging Motor
Researchers have taken long-term interest in the T4 virus, particularly because of the way the DNA double helix is packed extremely tightly within the viral head. As the DNA presses against the capsid walls, it generates high pressure (about ten times that of a bottle of champagne). This high pressure serves a functional purpose by driving the viral DNA into the host cell during the injection process.
The tight packing is achieved by a molecular machine called the DNA packaging motor. This motor binds to the opening of the empty capsid and translocates DNA into the capsid. The breakdown of ATP (a high-energy compound that liberates energy when key bonds within its structure are broken) powers this operation. When DNA is completely translocated, the packaging motor dissociates from the capsid.
As the video below shows, DNA is driven into the capsid when parts of the motor alternate between two distinct structural states. This motion, powered by ATP breakdown, generates an electrostatic force that pushes the highly negatively charged DNA molecule into the capsid.
Virologists had assumed the DNA packaging motor would bind only to empty capsids. The latest work, however, indicates that the motor will bind to full capsids as well, jamming even more DNA into the viral head.
This behavior is advantageous for the virus. Occasionally, the DNA packaging motor will prematurely debind from the capsid before DNA translocation has been fully achieved. When this happens, the resulting viral particle will lack a complete genome. Despite the premature disassociation, the indiscriminate binding of the DNA packing motor still allows the opportunity for a viral particle to be fully assembled. When the DNA packaging motor binds to a partially filled virus it will add more DNA into the viral head until the full complement of DNA is translocated into the capsid. The promiscuous behavior of the DNA packaging motor can be viewed as an elegant design feature, ensuring the maximum number of viruses are assembled.
This behavior also leads to viral particles with over-filled capsids, which generates even higher than normal pressures within the capsid and, thus, improves the efficiency of DNA injection into the host cell.
The Biomedical Uses of T4 DNA Packaging Motor
The Catholic University of America scientists realize that the DNA packaging motor’s promiscuous behavior could be exploited for biomedical applications, specifically gene delivery to targeted cells in the human body. They propose that capsid proteins could be altered to bind with a specified target cell and that the DNA packaging motor could be used to load up the modified capsid with pieces of DNA that contain genes useful for a variety of therapeutic purposes.
The T4 DNA Packaging Motor and the Case for Design
As I discussed last week, and spell out in detail in The Cell’s Design, the stark resemblance between man-made machines and molecular motors invigorate Paley’s Watchmaker argument and help make the case that life stems from the work of a Creator.

Some criticize the “new” Watchmaker argument by asserting the analogy between biomolecular machines and human designs is purely metaphorical and does not reflect a true relationship. As such, they maintain, the similarity between biomolecular machines and human designs cannot be used to make the case for intelligent design.2
The proposal by the scientists from the Catholic University of America, however, helps to formulate a response to this challenge. The potential use of the DNA packaging motor to package modified capsids with therapeutic pieces of DNA for delivery to specific cells and tissues highlights this biomolecular complex as a true machine. In fact, that is precisely how these researchers view the DNA packaging motor, as a machine. It is also provocative that the T4 DNA packaging motor’s architecture and operation inspired the design of a potential gene delivery system. In other words, the proposed use of the T4 DNA packaging machine as a machine affirms the Watchmaker argument.
The use of viruses to provide gene therapy has additional theological implications. Instead of representing a type of natural evil, viruses could be understood as a providential part of God’s creation.
Endnotes
- Zhihong Zhang et al., “A Promiscuous DNA Packaging Machine from Bacteriophage T4,” PLoS Biology 9, no. 2 (February 2011): e1000592; doi:10.1371/journal.pbio.1000592.
- Massimo Pigliucci and Maarten Boudry, “Why Machine-Information Metaphors are Bad for Science and Science Education,” Science and Education 20 (2011): 453–71; doi 10.1007/s11191-010-9267-6.





