Endogenous Retroviruses Help Fight Tumors
I recently watched the trailer for Eternals, an upcoming movie release from Marvel Studios. A creation of legendary comic writer and artist Jack Kirby, the Eternals made their first appearance in Eternals #1, published by Marvel Comics in July 1976.
As a race of humanlike beings created by aliens called the Celestials, the Eternals possess godlike powers. The Celestials created the Eternals to serve as Earth’s guardians, protecting humans from the Deviants, who are also a creation of the Celestials. Sometimes referred to as “mutates,” the Deviants have a humanoid form but are hideous in appearance. The Deviants are the sworn enemies of the Eternals.
The movie takes place after the events of Avengers: Endgame. The sudden return of half the Earth’s population triggers the “emergence.” As a result, the Deviants pose a renewed threat and the Eternals must reunite to protect humanity.
A Biochemical Superhero
Superheroes aren’t just found in the pages of the comics or on the big screen. They are also found in biochemical systems. And if there is a biomolecule that plays the role of a superhero, it would be p53, a protein that has been nicknamed the “guardian of the genome.”
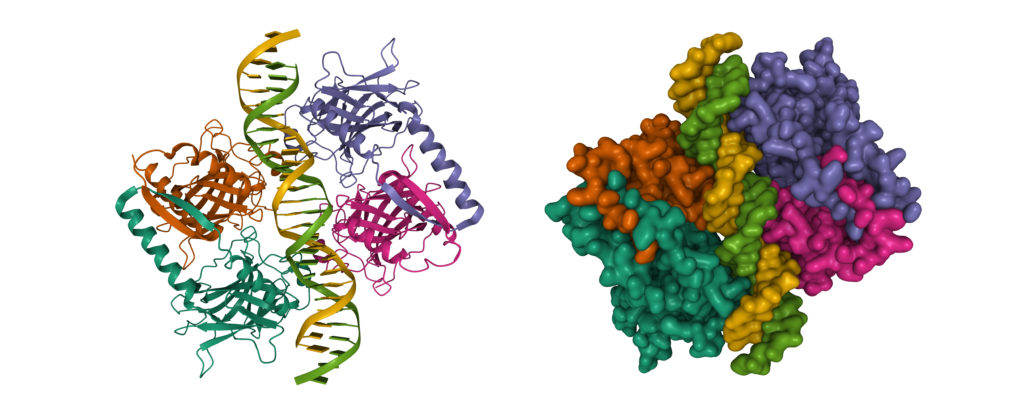
Figure 1: The Structure of p53
Credit: Shutterstock
The p53 protein has attained heroic status for several reasons. It preserves genome stability by binding to damaged DNA and activating DNA repair proteins. It can also exert its influence by arresting the cell cycle when the cell transitions between the G1 (cell growth) and S (DNA synthesis, readying the cell for replication) phases. By holding the cell at this stage in the cell cycle, p53 gives the DNA repair proteins enough time to clean up the damaged DNA before DNA synthesis takes place. If the DNA damage is too severe for repair, p53 can also initiate apoptosis (programmed cell death).
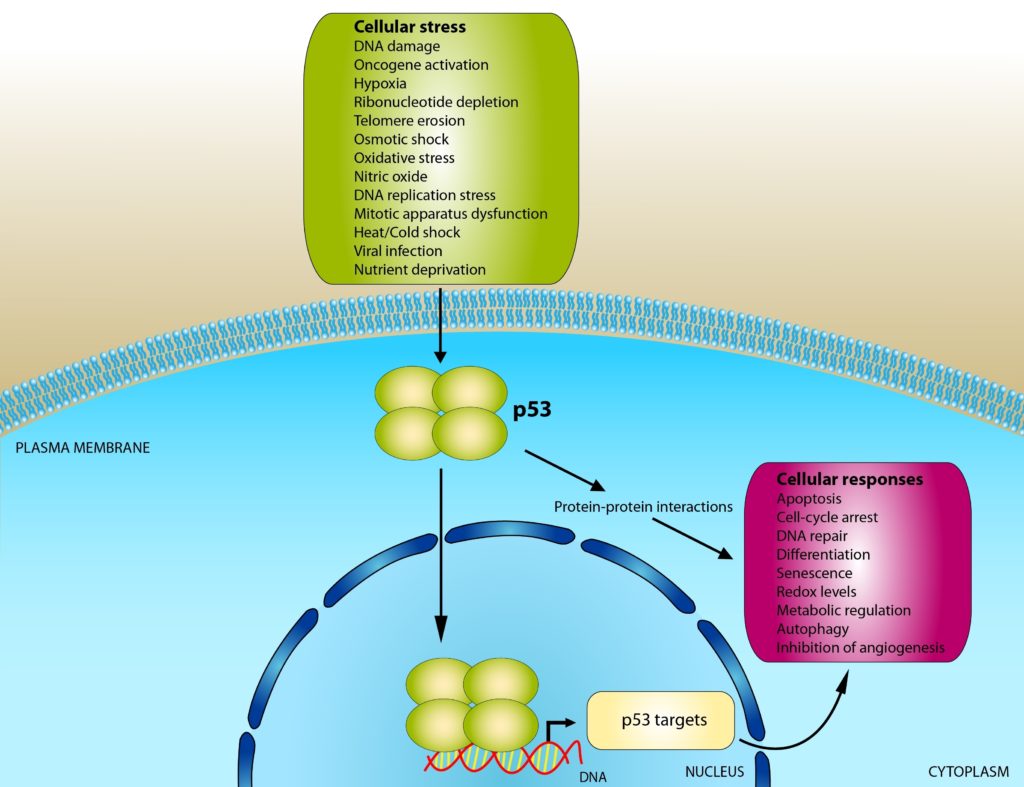
Figure 2: p53’s Mechanism of Action
Credit: Shutterstock
Some of p53’s other important roles include stem cell maintenance and tumor suppression. If p53 isn’t available, cells can readily become tumorigenic. Nearly 50% of the cells recovered from tumors display either a mutation or a deletion of the gene that encodes p53.
New insight into p53 function shows that, in addition to guarding the genome (and playing other heroic roles), this protein also helps defend the RTB genomics creation model from one of its most serious threats—the widespread presence of endogenous retroviral (ERV) sequences in genomes. (As a case in point, 8% of the human genome consists of ERV sequences.) The RTB genomics creation model asserts that the genome reflects the Creator’s handiwork, but for many people, ERVs undercut that claim.
So, how does p53 help rescue the RTB genomics model?
A brief discussion of the relationship between p53 and ERVs provides the necessary insight to address this question.
p53 Suppresses the Expression of ERVs
One way that p53 protects the genome is by suppressing the expression of ERVs. If not suppressed, ERVs sequences threaten the integrity of the genome. Many biologists believe that ERVs are the leftover remnants of a retroviral infection. Once incorporated into an organism’s genome, retroviral DNA is called an endogenous retrovirus. Initially these sequences remain active, producing new viral particles. However, if the ERV DNA suffers severe mutations, it can become disabled, remaining in the genome as nonfunctional, “junk” DNA.
ERVs possess an interesting property called retrotransposition. This capacity allows ERVs to make copies of themselves that, in turn, insert themselves elsewhere in the genome. For this reason, life scientists refer to ERVs as mobile DNA elements. ERVs are also called transposons, or retrotransposons. For ERVs, the process of retrotransposition begins when the ERV sequence is transcribed by the host cell’s machinery into RNA. Next, an enzyme known as a reverse transcriptase converts the ERV RNA into DNA, which can then be randomly inserted into the host cell’s genome through the activity of another enzyme known as an integrase. This insertion event can damage the genome by disrupting DNA sequences that encode proteins or DNA sequences that play a regulatory role.
Normally, p53 works in conjunction with the proteins LSD1 and DNMT1 to suppress ERV expression. This suppression prevents ERV sequences from undergoing retrotransposition. Life scientists have observed that 30% of p53 binding occurs at ERV sequences. Presumably, this binding is related to p53’s suppressive role.
Unlikely Partners: p53 and ERVs
Because of their capacity to damage the genome, ERVs typically are framed by biologists as the bad guys in our tale, threatening the integrity of the genome. But is that really the case?
While pursuing an anticancer therapy, a research team from Sweden discovered something quite unexpected when it comes to p53’s role in protecting the genome: it actually teams up with ERVs to activate the immune system, causing the immune system to attack villainous tumor cells that are disguised as ordinary cells.1 The researchers exposed three different types of cancer cells (melanoma, osteosarcoma, and breast cancer) in vitro to compounds that inhibit the proteins MDMX and MDM2. Normally, these proteins inhibit p53. The team reasoned that if these two proteins were inhibited, p53 could be activated to trigger an anticancer response. To the researchers’ surprise, they discovered that activation of p53 led to the expression of ERV sequences. In turn, the accumulation of ERV RNA in the cancer cells triggered the interferon response in these cells. This response regulates immune responses to pathogens and tumor cells.
Triggering the interferon pathway made it appear as if the cancer cells were infected with a virus even though they weren’t—a mechanism dubbed viral mimicry. Presumably, the process of viral mimicry would flag the otherwise “invisible” tumor cells for destruction by the immune system. The researchers discovered that biopsies taken from patients treated with the MDM2 and MDMX inhibitors showed evidence that cytotoxic CD8+ cells had, indeed, infiltrated the tumor.
The researchers propose a model to explain how p53 can paradoxically both repress and enhance the expression of ERV sequences. They argue that when cells are unstressed, p53 binds to ERV sequences in the genome and, working in conjunction with LSD1 and DNMT1, represses these sequences. In stressed cells, the levels of LSD1 and DNMT1 fall, leading to the expression of ERV sequences by p53 binding.
This biochemical insight may pave the way for improved anticancer therapies. It also validates a key prediction of the RTB creation model for genome structure and function and, in doing so, provides a response to one of the most significant challenges to the RTB model that stems from the presence of ERV sequences in the human genome and genomes of other organisms.
Endogenous Retroviruses and the Case for Human Evolution
For many people, the presence of ERVs in genomes is impossible to explain from a creation model perspective—without a whole lot of intellectual gymnastics. For many people, the distribution of ERVs in the genomes of humans and great apes convinces them that humans evolved from the same ancestor that produced the great apes.
Many human ERVs are also found in the genomes of chimpanzees, bonobos, gorillas, and orangutans. Not only do these ERVs share many of the same sequence patterns, but they also appear in corresponding locations in the genomes.
Evolutionary biologists explain this data by assuming that the shared ancestor of humans and chimpanzees, for example, became infected by these specific retroviruses. They believe that later, these ERVs underwent mutations that disabled them in this ancestor organism’s genome. The ERV sequences were then retained in the genomes of humans and chimpanzees as their separate evolutionary lineages diverged from the common ancestor. According to the model, the ERVs shared by humans and chimpanzees represent the molecular artifacts of infections that occurred millions of years ago and left their imprint on contemporary genomes via this (presumed) shared ancestor.
ERVs: Common Descent or Common Design?
Although most life scientists regard the shared biological features—including DNA sequences—possessed by organisms (that naturally cluster together) as evidence for their shared evolutionary ancestry, I argue that it is possible to advance an alternative explanation for biological similarities. Instead of evincing common descent, these similarities could be interpreted as shared biological designs with the mutual features as manifestations of a common blueprint—an archetype that arises out of the Creator’s mind.
The RTB creation model interprets the shared features in the genomes of organisms as manifestations of genomic archetypes. In other words, the genetic similarities in the genomes of humans and the great apes—including so-called junk DNA sequences—were intentionally introduced by the Creator.
To justify this interpretation, the shared genomic features must serve a function.
And, indeed, this is the case for ERVs (and the other types of junk DNA).
The viral mimicry mechanism that occurs in tumor cells when p53 becomes activated is largely possible because of the similarity between these ERV and retroviral sequences. This requirement explains why a Creator would introduce genetic elements into the human genome (and the genomes of other creatures) that share sequence elements with viruses.
So, far from being the villain of this tale, ERV sequences turn out to be p53’s faithful sidekick in the battle against cancer.
Resources
Thinking about Evolution by Anjeanette Roberts, Fazale Rana, Sue Dykes, and Mark Perez (book)
Who Was Adam?, 2nd exp. ed., by Fazale Rana with Hugh Ross (book)
The Cell’s Design by Fazale Rana (book)
Endogenous Retroviruses Have Function
“Koala Endogenous Retroviruses (ERVs) Protect against Retroviral Infections” by Fazale Rana (article)
“SARS-CoV-2 Biology Points to Endogenous Retrovirus Design” by Fazale Rana (article)
“Endogenous Retroviruses (ERVs) Protect Early-Stage Human Embryos” by Fazale Rana (article)
“Endogenous Viral Elements (EVEs) Assist Wasps in Parasitizing Their Host” by Fazale Rana (article)
“Questioning Evolutionary Presuppositions about Endogenous Retroviruses” by A. J. Roberts (article)
“A Common Design View of ERVs Encourages Scientific Investigation” by A.J. Roberts (article)
The Historical and Philosophical Case for Common Design
“Archetype or Ancestor? Sir Richard Owen and the Case for Design” by Fazale Rana (article)
“Duck-Billed Platypus Venom: Designed for Discovery” by Fazale Rana (article)
“Does Old-Earth Creationism Make God Deceptive?” by Fazale Rana (article)
The Negative Impact of the Junk DNA Concept on Scientific Advance
“Does the Evolutionary Paradigm Stymie Scientific Advance?” by Fazale Rana (article)
“Evolution’s Flawed Approach to Science” by Fazale Rana (article)
“Does Evolutionary Bias Create Unhealthy Stereotypes about Pseudogenes?” by Fazale Rana (article)
Endnotes
1. Xiaolei Zhou et al., “Pharmacological Activation of p53 Triggers Viral Mimicry Response Thereby Abolishing Tumor Immune Evasion and Promoting Anti-Tumor Immunity,” Cancer Discovery (July 6, 2021): OnlineFirst, doi:10.1158/2159-8290.CD-20-1741.