Cone Cell Mitochondria Focus Attention on Eye Design
I have become painfully aware that my vision is beginning to decline. Objects at a distance appear blurry to me and I need glasses to read. It’s all part of the aging process, I guess.
In light of my failing eyesight, it’s easy for me to forget how remarkable human vision actually is.
- Did you know that we can distinguish around one million different colors?
- Did you know that our rod cells can respond to a single photon of light?
- Did you know that on a clear, dark night humans can spot a single candle flame as far away as 30 miles?
In fact, there is no limit to how far we can see provided the object emits or reflects photons of light.
As remarkable as our vision is, there are animals with even better vision. Take eagles, as a case in point. Their vision has better resolution than human vision. These birds of prey see objects more clearly than we do at eight times the distance. For example, they can see a small camouflaged animal from two miles away, something we can’t do. Eagles can also see a wider range of colors than we can, allowing them to detect hidden prey.
Ironically, many skeptics regard the vertebrate eye as the quintessential example of a flawed biological design and, hence, prima facie evidence for an evolutionary history for life. Yet, the more we learn about the inverted retina, the deeper the rationale for its peculiar design becomes. New insights from a team of researchers from the National Eye Institute in Bethesda, MD, illustrate this point.1
Design of the Human Eye Factors into the Creation-Evolution Debate
In a Scientific American piece, Trevor Lamb writes:
The human eye is an exquisitely complicated organ. It acts like a camera to collect and focus light and convert it into an electrical signal that the brain translates into images. But instead of photographic film, it has a highly specialized retina that detects light and processes the signals using dozens of different kinds of neurons. So intricate is the eye that its origin has long been a cause célèbre among creationists.
The eye, far from being a perfectly engineered piece of machinery, exhibits a number of major flaws—these flaws are the scars of evolution. Natural selection does not, as some might think, result in perfection. It tinkers with the material available to it, sometimes to odd effect.2
Many life scientists readily point to the inverted structure of the vertebrate retina as its chief flaw. The rod and cone cells responsible for detecting the light that falls onto the retina’s surface are oriented away from the light source, not toward it as most people would reasonably assume. In The Blind Watchmaker, evolutionary biologist Richard Dawkins insists, “Any engineer would…laugh at any suggestion that the photocells might point away from the light, with their wires departing on the side nearest the light. Yet this is exactly what happens in all vertebrate retinas. Each photocell is, in effect, wired backwards.”3
Despite these types of pronouncements, careful examination of the anatomy and physiology of the vertebrate eye and—specifically, the inverted retina—reveals a deep-seated rationale for its design, a rationale that undercuts the complaints of skeptics and evinces a Creator’s handiwork.
Before I describe the details of the National Eye Institute team’s findings, it may be helpful to offer a little bit of background about the vertebrate eye, the vertebrate retina, and the rod and cone cells found in the retina.
Design of the Vertebrate Eye
Vertebrates have eyes that work very much like a camera (hence, they are called camera eyes. (See figure 1). The outermost surface of the eye, the cornea consists of a transparent set of layers made up of epithelial cells and connective tissue. The cornea forms a covering over the anterior chamber, iris, and pupil. The anterior chamber, which is the space between the cornea and the iris (and pupil), is filled with a water-like fluid called the aqueous humor). The thin, colorful iris is annular and is attached to muscles that open and close the pupil in response to the amount of light impinging upon the eye.
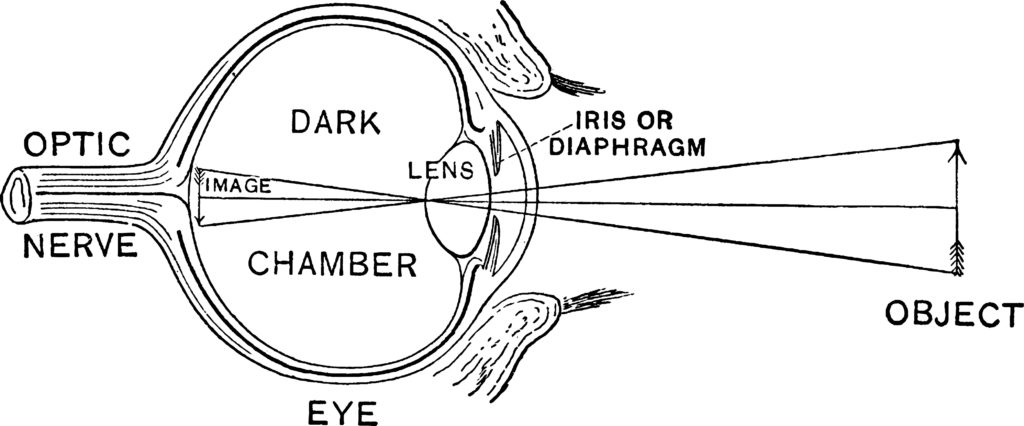
Figure 1: The Camera Eye of Vertebrates
Credit: Shutterstock
Light passes through the cornea, the aqueous humor, and the pupil to the lens, which focuses light on the inner surface of the eye, called the retina. Like the cornea, the lens consists of layers of cells and connective tissue. A collection of ligaments suspends the lens in place. After passing through the lens, light is transmitted through the vitreous humor, the colorless gel-like substance filling the eye’s dark chamber. The cornea and the lens work in tandem to focus light on the surface of the retina. Specialized photoreceptor cells in the retina absorb photons of light and, in turn, generate electrical impulses that the optic nerve transmits to the brain.
Structure of the Rod and Cone Cells
Photoreceptor cells fall into two categories: rods and cones. Both cell types possess the same basic structure, with rod cells displaying a more elongated shape than cone cells (see figure 2). These cells have several distinct regions. The central region contains the cell body that houses the nucleus. Extending from one of the poles of the cell body are the inner and outer segments. The inner segment is packed with mitochondria, responsible for generating the energy needed to support the intense metabolic activity that takes place in the outer segment.
The outer region of the cell consists of stacks of membranes. Embedded in the membrane stacks are a large number of copies of the protein called opsin. Associated with opsin is a co-enzyme called retinal. Together opsin and retinal form a complex called rhodopsin in rod cells and conopsin in cone cells. Rhodopsin and conopsin absorb photons of light, triggering a biochemical cascade that prevents the release of a neurotransmitter at the synaptic body, which extends from the opposite pole of the cell body.
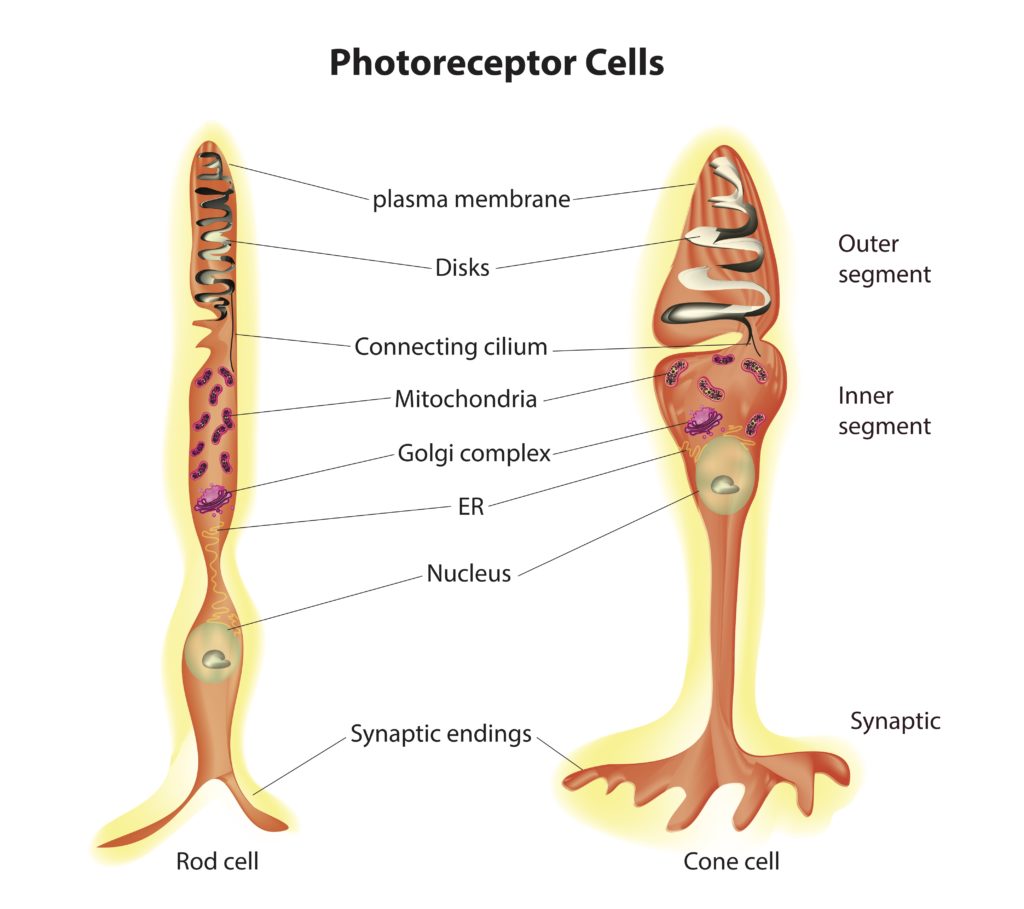
Credit: Shutterstock
The synaptic body forms a synaptic connection with a neuron called a bipolar cell (see figure 3). This neuron has two projections that extend from opposite poles of the cell.
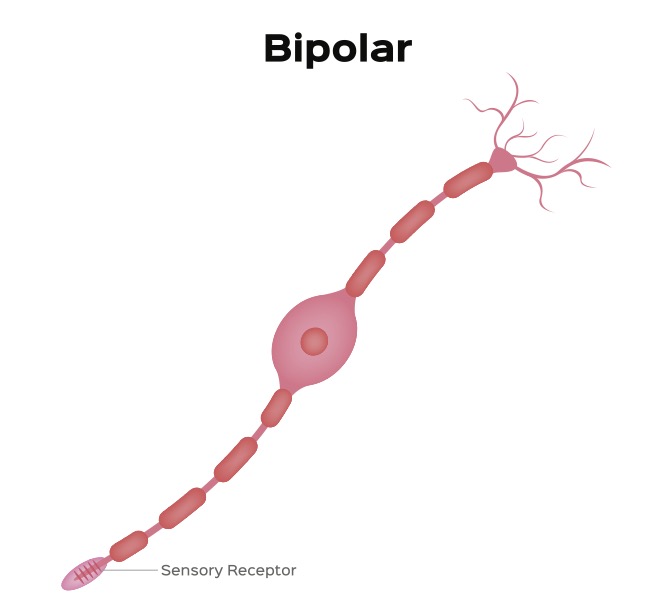
Figure 3: A Bipolar Neuron
Credit: Shutterstock
When the rod or cone cell is in a resting state, it releases a neurotransmitter at the synaptic junction with the bipolar cells. This activity prevents the bipolar cell from releasing its own neurotransmitters. When the rod or cone cells absorb light, it causes them to stop releasing their neurotransmitter. This change causes the bipolar cells to release their neurotransmitters, which excites the synapses between the bipolar cell and the ganglia (a group of nerve cells) that, in turn, transmit an electrical signal along the optic nerve to the brain.
The Inverted Retina
The retina consists of layers of neural cells that include 6 to 7 million cone cells and about 90 to 100 million rod cells. The innermost layer of the retina is composed of epithelial cells. The next layer is made up of rod and cone cells. Rod cells primarily provide black-and-white vision, operating under dim light conditions. Cone cells are responsible for color vision and function under bright light conditions.
Biologists describe the vertebrate retina as inverted because the outer segments of the photoreceptors (which absorb photons of light) are oriented away from the retinal surface and the source of the light (see figure 4). The outer segments juxtapose the epithelial cells of the pigmented retinal epithelium. The bipolar cells form the cell layer that sits on top of the layer formed by the photoreceptors. The outermost cell layers of the retina consist of the neurons that comprise the ganglia that coalesce into the optic nerve.
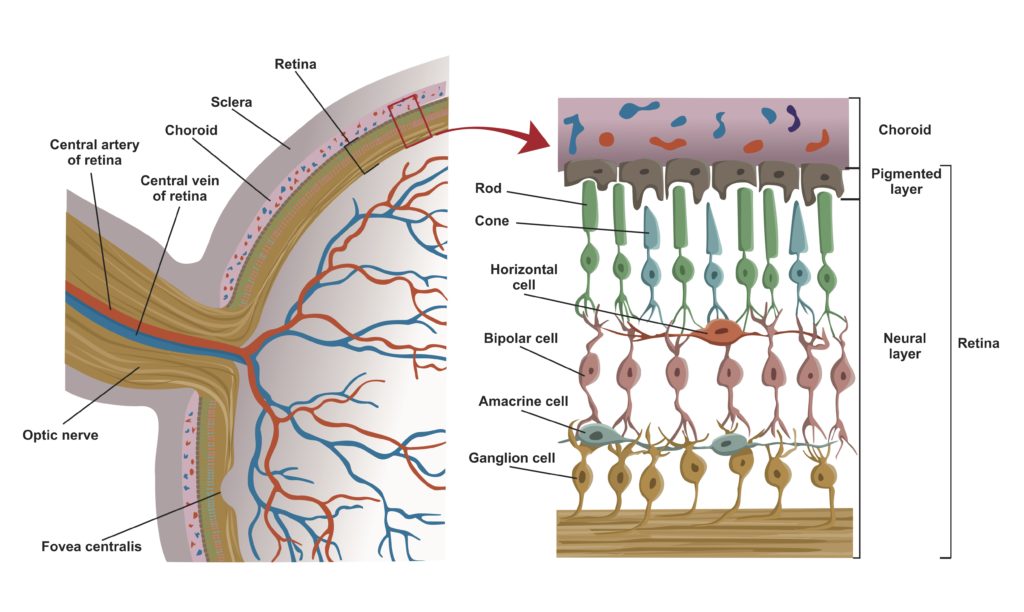
Credit: Shutterstock
Many skeptics question the design of the inverted retina for several reasons. Not only are the outer segments of the rod and cone cells orientated away from the light source, but cellular materials (such as the inner segments and the cell bodies of photoreceptors, along with the bipolar cells and ganglia) occupy the space between the membrane stacks of the outer segments and the light source. This intervening material blocks and scatters the light before it can reach the outer segments.
Adding to these two “flaws” is the blind spot in the field of vision that results from the optic nerve punching a hole near the center of the retina. This configuration is a consequence of the inverted design of the retina. The optic nerve forms from the coalescing ganglia that reside at the surface of the retina. Once the optic nerve forms, it needs to punch through the retina to make it to the brain.
Even though it might be tempting to conclude that the vertebrate retina is plagued by flawed designs, life scientists have discovered features of the vertebrate retina that indicate its design may be far more elegant than commonly thought. For example, researchers have discovered compensatory design features that circumvent the light scattering problems caused by the materials that reside between the retinal surface and the outer segments of the photoreceptors.
Compensatory Designs in the Vertebrate Retina
In February 2021, investigators from the National Eye Institute uncovered one more example of a compensatory design. These researchers learned that the mitochondria in the inner segment—which help supply the intense energy needs of the biochemical processes that take place in the outer segment—interact to form an organelle complex that functions as a microlens, concentrating the light that passes through the inner segment on the membrane stacks of the outer segment. So, instead of interfering with light transmission, the mitochondria reduce light scattering based on the architecture of the subcellular structure that results from mitochondrial interactions. The investigators also discovered that the mitochondrial complex creates an angular dependence of the light intensity as it makes its way to the outer segment. The light near the center of the beam displays a greater intensity than light near the edges. This effect improves visual resolution because this type of angular dependence reduces optical aberrations that would occur near the outer edge of the light beam.
This discovery isn’t the first of its type. In 2009, researchers from Germany learned that the arrangement of chromatin in the nucleus (which is housed in the cell body) forms a microlens in nocturnal animals that focuses low light levels onto the outer segment.4 Again, the nucleus helps capture light, rather than scattering it.
Researchers have also discovered that radial glial cells associated with the retina function as optical fibers. That is, the radial glial cells (star-shaped cells that help maintain the structure of nervous tissue and transport nutrients to neurons) form fibers oriented in the direction of light propagation through the retina. This function allows them to transmit light efficiently from the surface of the retina to the photoreceptors. Radial glial cells have a higher refractive index than the surrounding tissue matrix, serving as a low-scattering conduit for light and thus transmitting images capably and with little distortion.
Elegant Design of the Retina
These insights build upon earlier studies that point to a rationale for the inverted design of the vertebrate retina. For example, the vertebrate eye functions like a premium quality camera. Yet vertebrates’ high-sensitivity, high-resolution vision comes with a steep metabolic cost. On a per weight basis, the retina is one of the most metabolically demanding tissues in vertebrates. Relatively high levels of nutrients and oxygen must be supplied to the rod and cone cells to sustain the metabolic processes that undergird light absorption and the metabolic cascades that trigger the electrical impulses initiated by the rod and cone cells.
A bed of capillaries located in the choroid supplies these nutrients and oxygen. The choroid is positioned on one side of the retinal pigmented epithelium and the outer segments of the rods and cones on the other. The proximity of the choroid capillaries and the outer cell segments makes it possible for simple diffusion of oxygen and nutrients to supply the metabolic needs of the outer segments of the rod and cone cells. Moreover, the choroidal capillaries also allow for the efficient removal of waste products generated by the metabolic processes in the outer segments of the cone and rod cells. These capillaries also serve as a sink, removing heat from the outer segments. The intense metabolic activity in the outer segments generates a significant amount of heat, which, if not efficiently dissipated, will cause the proteins in the outer segment to denature.
It is true that orienting the outer segments of the rod and cone cells toward the light source would dramatically reduce light scattering. However, this orientation would also limit the number of cone and rod cells in the retina because it would hamper efficient oxygen and nutrient supply, waste removal, and heat dissipation. Returning to the camera analogy, imagine each rod and cone cell as a pixel. For digital cameras, the number of pixels correlates to the camera’s resolution. Likewise, limiting the number of rod and cone cells in the retina would reduce the resolution of vertebrate vision.
The sensitivity of the vertebrate eye arises from metabolic cascades that amplify the biochemical response when a rhodopsin or conopsin molecule absorbs a single photon. Again, if the photoreceptors were oriented toward the light source, the resulting inefficiencies in nutrient and oxygen delivery and heat and waste removal would limit the capacity of rod and cone cells to mount a sufficient metabolic response to be able to amplify the biochemical processes initiated by the absorption of a small number of photons.
Far from causing engineers to laugh, this rationale highlights a design elegant enough to inspire awe among technologists and life scientists alike.
Even though objects in my field of view have become blurrier as I get older, scientific advance during my lifetime continues to bring the remarkable design features of the vertebrate eye into sharper focus. Instead of the vertebrate eye being a cause célèbre for evolutionary biologists, the rationale that undergirds all of the design features of the inverted retina bespeak a Creator’s handiwork.
Resources
“Design with a Purpose” by Brad Sargent (article)
“Is the Design of the Human Retina Sensible?” by Eddie Del Rio (article)
“How We Keep Our Eyes on Target?” by Eddie Del Rio (article)
“Your $45,000 Eyes” by Curt Deckert (article)
“Seeing the Wonder of Transparency” by Nick Tavani (article)
“Optical Illusion Exposes the Reality of Design” by Fazale Rana (article)
“New Research Highlights the Elegant Design of the Inverted Retina” by Fazale Rana (article)
Endnotes
- John M. Ball, Shan Chen, and Wei Li, “Cone Mitochondria Act as Microlenses to Enhance Light Delivery and Confer Stiles-Crawford-Like Direction Sensitivity,” BioRxiv (February 12, 2021); doi:10.1101/2021.02.11.430818.
- Trevor D. Lamb, “Evolution of the Eye,” Scientific American 305 (July 2011): 64-69.
- Richard Dawkins, The Blind Watchmaker: Why the Evidence of Evolution Reveals a Universe without Design (New York: W.W. Norton, 1996), 93.
- Irina Solovei et al., “Nuclear Architecture of Rod Photoreceptor Cells Adapt to Vision in Mammalian Evolution,” Cell 137 (April 17, 2009): 356–368, doi:10.1016/j.cell.01.052.






