Should We Eat Lab-Grown Meat? A Christian Perspective
It’s the quintessential meat lover’s dish and a holiday favorite, popularized by sportscaster John Madden and Cajun chef Paul Prudhomme.
Turducken, a blended word combining turkey, duck, and chicken, consists of a deboned chicken stuffed into a deboned duck that, in turn, is stuffed into a deboned turkey. The cavity of the chicken and the gaps between the birds is filled with a bread crumb and sausage mixture. Alternatively, some cooks add pork or veal roast to the chicken cavity instead of traditional stuffing.
Turducken epitomizes our love affair with meat and demonstrates that there is no distance a true meat lover won’t traverse to satisfy his or her cravings. More and more people around the world are becoming meat lovers. But this love affair may not last long because many experts believe that the world’s meat producers won’t have the wherewithal to satisfy the ever-increasing demand.
Global Growth of the Demand for Meat
The yearning for meat (and other animal products such as milk and eggs) has grown worldwide. In 1960, 71 million tons of meat was produced (and consumed), globally. By 2022, that number had swelled to 340 million tons. Some projections put global meat production between 460 million and 570 million tons by 2050.1
The increased demand for meat is due to two main factors: (1) a growing worldwide human population, and (2) increased affluence in the developing world. As people acquire more wealth, their diets include more meat. At this current pace, there’s a growing concern that farmers won’t have the production capacity to satisfy the worldwide demand.
Environmental Impact of Meat Production
Another problem concerns the now widely held belief that the growing demand for meat will inevitably cause increasing harm to the environment.
By some estimates, animal agriculture generates about 18% of the worldwide greenhouse gas emissions. Waste from animal agriculture also leaches into water supplies, particularly when a lot of animals are raised in a concentrated space. This waste runoff creates dead zones in the affected water supplies.
Animal agriculture also requires extensive land use. The increased demand for meat has become one of the drivers of deforestation. According to some estimates, about 1 acre of forest land is cleared every second. And much of it is used for animal agriculture. About 91% of the deforested land of the Amazon rainforest is now used to raise livestock for meat production.
Land use for meat production is inefficient. The amount of land it takes to produce a gram of edible beef protein is about 20 times greater than what it takes to produce a gram of edible proteins from beans and lentils.
In the US, 67% of the crops produced on farmland goes to feed animals raised for meat production. Only 27% of crop production provides calories directly consumed by human beings. In addition, half of the water used in the US is for animal agriculture.
In short, animal agriculture puts a strain on limited natural resources and causes environmental damage. This strain and damage will only get worse as more and more people consume meat.
Meat Production and Animal Cruelty
There’s also an ethical dimension to the increased global demand for meat. To sustain the current demand, around 70 billion land animals and trillions of marine animals are slaughtered each year. The most efficient way to grow this number of animals is through factory farming, which many people find to be cruel and inhumane.
Lab Meat as an Alternative
In the face of the limited resources available to increase meat production, concerns for the environment, and concerns about animal welfare, some biotechnologists are exploring another option: the production of meat in the laboratory from stem cells.
Advocates claim that lab meat would be friendlier to the environment than factory farming and would bring an end to the cruelty of factory farming. And it could help supply the world’s growing desire to consume more meat with a healthier, more versatile product than meat produced from animal agriculture.
Thanks to advances in synthetic biology and tissue engineering, researchers can now “grow” meat in the laboratory from a few stem cells recovered from tissue biopsies taken from animals. This lab-grown tissue is called lab meat, cultured meat, or synthetic meat. But most advocates prefer the less unpleasant label, clean meat.2
Technologists working in this field hope that they can refine laboratory protocols to be efficient enough that lab meat can be produced at large scales and low enough cost that it could be sold in grocery stores and other food markets. If this objective can be achieved, then this emerging biotechnology may become one of the most economically advantageous technologies, impacting the agriculture, grocery, and food service industries.
Growing Meat in the Lab3
A source of stem cells is required to grow meat in the lab. Because stem cells can be cultured into “immortal” cell lines, once established, the cell line can serve as an ongoing source of cells for meat production. Estimates communicated in popular media outlets routinely claim that one biopsy could generate tons of meat products. More measured estimates indicate that with current technology, lab meat production will dramatically reduce the number of animals required for meat production, but not to the extent popularly claimed. A recent study focusing on beef indicated that wide-scale lab meat production could reduce the number of cattle needed to produce beef globally by a factor of four hundred.4 By optimizing the biopsy process and improving the efficiency of stem and progenitor cell recovery from the biopsies, researchers hope the advances could lead to an even greater reduction in animals used for meat production.
Investigators have tried several different types of stem cells to culture meat, and the most promising have been muscle stem cells (called myosatellite cells). These cells are humanely isolated by taking a muscle biopsy from an anesthetized animal, causing the creature minimal discomfort. Once the tissue biopsy has been secured, the structure of the tissue is disrupted by an enzymatic treatment. The tissue disruption frees the cells from other tissue components, making it easier to isolate and recover them.
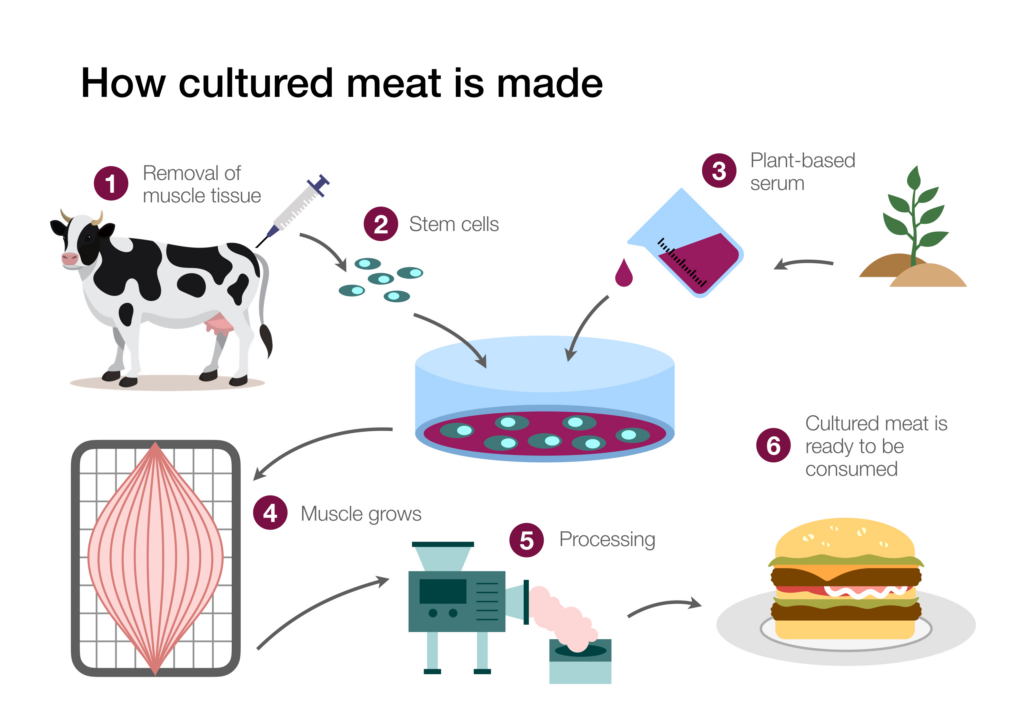
Figure 1: The Growth of Meat in the Lab
Credit: Shutterstock
Biotechnologists have a good understanding of the growth and development process that turns myosatellite cells into muscle fibers. Myosatellite cells have the capability of dividing and proliferating in cell culture. Under the right culture conditions, these cells can be encouraged to transform into myogenic progenitor cells (or myoblasts). In turn, the myoblasts can be coaxed further to differentiate into myocytes. Afterward, these cells fuse together to form a fibrous-like “supercell” that possesses multiple nuclei. This fused cellular structure, called a myotube, can mature into a myofiber that interacts with other myofibers to form a muscle fiber. (See figure 2.)
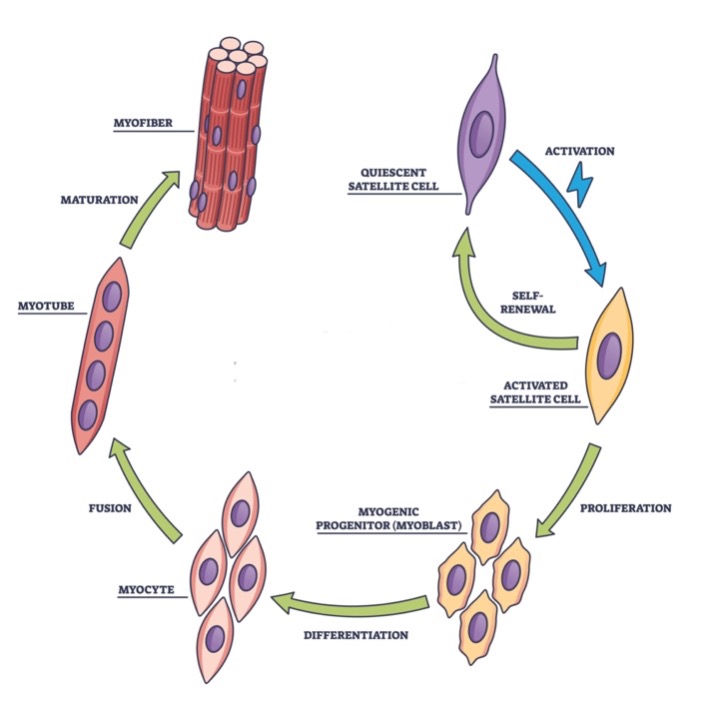
Figure 2: The Growth of Muscle Fibers from Myosatellite Cells
Credit: Shutterstock
With myosatellite cells in hand, researchers first culture them in two-dimensional cell cultures using fetal bovine serum (FBS) as the cell culture medium. (One area of active research involves finding a suitable replacement for the controversial use of FBS.) Researchers use precise culture conditions to induce the myosatellite cells to develop into myoblasts, which includes the addition of the right growth factors (a collection of proteins that regulate specific stages of cell growth and differentiation). Next, the myoblasts are transferred into bioreactors designed to grow three-dimensional cell cultures that will eventually form the lab meat.
During this stage, scaffolding is introduced to provide the three-dimensional framework for the lab meat. Scaffolding refers to edible material that supports the organization of meat cells into the desired shape. The design of the scaffolding is key. It must possess the just-right porosity. The pores allow for cellular waste products to diffuse away and allow nutrients and growth factors to reach the growing cells. If the porosity isn’t right, necrotic (dead zones of cells) will develop in the cell culture interior. The scaffolding must have the just-right biochemical properties as well. The cells must adhere to the scaffolding through chemical interactions between the scaffolding material and the cell surface. The scaffolding also needs to be capable of retaining water. It also must have the just-right rigidity to ensure that the three-dimensional shape of the cell culture is maintained. And, of course, the scaffolding must not detract from the flavor and texture of the lab meat.
Several different materials have been studied for scaffolding use, including cellulose, chitin, and collagen, which is the component that forms connective tissue that is naturally associated with living muscle.
As the myoblasts fuse to form myotubes in the bioreactors and then grow into myofibers, researchers must electrically and mechanically stimulate the myofibers. This stimulation helps promote cell proliferation and differentiation and the maturation of the myofibers.
During this stage, researchers also add other components (vitamins, minerals, colorants) to the cell culture, including adipocytes (fat-producing cells). The addition of adipocytes is particularly important because fat plays an important role in the flavor and texture of meat. Other added materials improve the aesthetics of the lab meat.
Once the growth of the cell culture is completed, researchers process the cell culture to mimic meat products (ground meat or sausages). The entire process takes between 2 to 8 weeks, depending on the type of meat being grown.
Commercial Viability
Even though the technology is in place, one of the big hurdles that has stood in the way of bringing lab meat to market is the cost. In 2013, the first burger made from lab meat—the creation of Mark Post of Maastricht University—was served at a press conference in London. The cost of that burger was estimated at over $300,000. But in the last decade, the cost of producing lab meat has plummeted. As of spring 2022, Post and his company, Mosa Meats, had reduced the cost to less than $10 per burger.5
Today, there are nearly 100 biotech companies around the world working to bring lab meat products to market. According to the Mackenzie Report, some estimates place the value of the global market for cultured meat at $25 billion by 2030, which, according to cost projections, will be the time when the cost of lab meat reaches parity with conventional meat.
A critical step in achieving this economic milestone came in the early summer of 2023, when food technology companies GOOD Meat and UPSIDE Foods received approval from the USDA to sell the first-ever lab meat poultry product made from animal cells.6
Health Benefits
Nutritional Value
Apart from the environmental benefits and the humane treatment of animals, some experts claim that lab meat would be healthier overall to consume than conventional meat. Lab meat would contain most of the nutrients of conventional meat products (proteins and fats). And those nutrients that are missing can be added to augment the nutritional value of lab meat. In addition, genetic engineering techniques could be used to modify the myosatellite cells or the adipocytes so that they produce a greater proportion of healthier fats (unsaturated fats and omega-3 fatty acids). Also, other nutrients that don’t naturally occur in meat could be added as well.
Fewer Antibiotics
Because animals are confined in small spaces during factory farming, farmers and ranchers must give the creatures antibiotics to curtail the spread of bacterial infections, thereby preventing animal suffering and sustaining meat production at high levels. These antibiotics make their way into the meat products. They also leach into the environment and contribute to the rise of antibiotic-resistant superbugs. It may be possible to produce lab meat without antibiotics, though some experts think that antimicrobials might be needed to prevent lab meat from being contaminated by bacteria and fungi during lab meat production.
Reduction in Food-Borne Illnesses
Lab meat should also dramatically reduce food-borne illness. When conventional meat is produced, it is exposed to intestinal contents and animal waste during animal slaughter and the early stages of meat processing. This exposure contaminates the meat with pathogens that can cause food poisoning in consumers.
Despite the potential health benefits, one concern that has been raised by critics is the use of hormones and growth factors to culture the lab meat. It is unknown if these added compounds will have negative short- and long-term effects on human health if lab meat is consumed on a regular basis. Today, the European Union prohibits the use of hormones to promote growth in animals raised for conventional meat production.7
Lab Meat and the Culinary Arts
Lab meat has also piqued the interest of chefs. Many are willing to serve lab meat in their restaurants for a variety of reasons, including food safety and environmental friendliness. But they’re also interested in lab meat because of the ability to control the flavor and texture of the product by changing culture conditions.8
Lab meat also holds the prospect of making exotic meats widely available. Because lab meat doesn’t require the destruction of an animal, biopsies of animals that are nondomesticated or on the verge of extinction can be used to culture meat. It’s also possible to produce meat by combining cells from different animal sources to produce a high-tech version of turducken.
Critics have pointed out that lab meat will likely disappoint, at least in the near term, by failing to provide chefs and consumers with the diversity of meats that comes from different animal breeds and the different cuts of meat currently available for conventional meat products. For now, lab meat will be sold and consumed primarily as a ground product.
Is Lab Meat Too Good to Be True? The Environmental Impact
Most of the publicity surrounding lab meat has been positive. Yet, a number of scientists and technologists have questioned if lab meat will deliver the benefits its advocates tout. A critical review article published in 2020 by two French scientists presents a balanced assessment of the benefits and costs of lab meat production. Their analysis shows that the claimed environmental benefits that come from producing lab meat compared to conventional meat offerings have been overstated.
Greenhouse Gas Emissions
For example, these scientists cite a study published in 2019 that demonstrates switching to lab meat and reducing conventional beef production will, indeed, reduce methane emissions by replacing them with carbon dioxide release.9 In the short term, this change will reduce global warming because methane is a more potent greenhouse gas than carbon dioxide. However, methane is much more short-lived in the atmosphere than carbon dioxide, meaning that in the long term lab meat production will raise, not lower, global temperatures.
Work by investigators from the University of California, Davis, draws a similar conclusion.10 Lab meat manufacture requires production facilities and an ongoing supply of raw materials (growth media, additives, and scaffolding). These raw materials must be manufactured, packaged, and transported to the production facility. The production of lab meat and its raw materials will require water and power. And it will generate waste.
Assuming current technology, the UC Davis team determined the carbon dioxide equivalents per kilogram of meat produced as lab meat and as conventional meat using animal agricultural practices. By determining the energy usage for each step of the process, they discovered that lab meat production generated 4 to 25 times more carbon dioxide than the production of conventional beef.
The UC Davis team determined that one of the chief culprits contributing to the environmental stress caused by lab meat production is the growth medium used to culture the cells and induce them to form myofibrils. This growth medium contains sugars, growth factors, salts, amino acids, and vitamins. Each of these components must be produced. The sugars come from crops. The growth factors must be extracted and purified from biological sources in a laboratory setting. All the components must be a high-purity pharmaceutical grade (approved by the FDA). This process requires expensive and energy-intensive purification steps.
Culturing animal cells is tricky business. These cell cultures are fastidious, making the purity grade of these materials essential. It’s the only way to ensure that no microorganisms and toxins find their way into the growth medium from the raw materials. If cell cultures become contaminated with microorganisms, the contaminating cells will outgrow the animal cells and overrun the cell culture with unwanted microbes. There’s no way to bypass the need for pharmaceutical-grade raw materials to culture cells for lab meat production.
Land Usage
The French scientists agree that the land required for animal agriculture far exceeds that needed for lab meat production. But they point out that livestock production provides beneficial environmental services. The manure generated from livestock provides organic matter, nitrogen, and phosphorus that maintains soil fertility. Also, much of the land used to raise cattle isn’t useful for any other purpose. So, while some of the land used for animal agriculture comes at a real environmental cost (for example, the conversion of Amazon rainforest to land used to raise cattle), much of it is nonarable grassland that is ideally suited for livestock production.
Is Lab Meat Too Good to Be True? The Economic Impact
Without question, lab meat production has the potential to be an economically beneficial technology. It will create a variety of new jobs. But these new jobs will come at a cost. The French scientists suggest that the switch away from conventional agriculture to lab meat production will likely devastate rural communities that rely mostly on agriculture as the primary source of income.
They also point out that animal agriculture may not diminish as much as people might hope. Even if laboratory operations become the primary means of meat production, livestock will still be needed to produce eggs, milk, wool, fiber, and leather.
Is Lab Meat Too Good to Be True? The FBS Problem
One of the primary justifications for lab meat production is the desire to mitigate animal cruelty through the reduction of factory farming and animal slaughter. Unfortunately, the use of FBS as the medium to culture myosatellite cells into muscle fibers all but eliminates any gains in animal welfare from lab meat production.
FBS is ideal as a cell culture medium because it contains vitamins, hormones, and growth factors needed to convert stem cells into differentiated cells. But FBS comes from the blood of cow fetuses. To harvest this blood, not only is the cow fetus destroyed but often the mother is slaughtered as well.
Currently, 800,000 liters of FBS are produced each year and that amount requires the destruction of 2 million cow fetuses. Of course, if wide-scale production of lab meat ensues, much more FBS would be required.
FBS is also expensive. A half-liter costs about $700 dollars. The need for FBS is one of the reasons lab meat costs so much to produce. For context, hundreds of liters of FBS are needed to generate just over 2 pounds of lab meat, which is enough to make 20 fast food hamburgers.
Other problems plague FBS usage for lab meat production, including batch-to-batch variations and the risk of microbial contamination.
The good news is that numerous biotech companies have found ways to reduce the amount of FBS required to produce lab meat or eliminate it altogether.11 This advance will undoubtedly contribute to the commercial viability of lab meat and truly make it a cruelty-free product.
Tentative Steps Forward?
As things stand now, the dish with lab meat is still in the oven. Nobody knows if it will be a half-baked biotechnology or not. It is unclear if consumers will accept lab meat as an alternative to conventional meat. And it is even less clear if lab meat will have the positive impact on the environment many of its advocates claim. Lab meat does hold the potential to be an economically rewarding technology. It will create jobs and open new economic opportunities. However, it will also change the face of animal agriculture, which may well harm some rural communities.
Even when it comes to animal cruelty, it’s not clear that lab meat will lead to the outcome everyone hopes—even if the FBS problem is solved. Meat is only one of the products produced by animal agriculture. Efficient egg and milk production, too, lends itself to factory farming. And other animal products like leather require animal sacrifice.
Still, it is a biotechnology well worth exploring. If it can realize—even in part—some of its promises, it will improve animal welfare and help us better care for the environment.
A Christian Perspective on Lab Meat
One final point of concern for Christians is, What should our response be to biotechnologies such as lab meat?
- Are we overstepping our God-given bounds when we develop biotechnologies such as lab meat?
- Are we operating outside God’s design?
- Are we playing God?
The biblical passages most relevant to these questions are the human creation accounts found in Genesis 1 and 2. This section of Scripture teaches that human beings are uniquely made in God’s image. With this special status comes certain responsibilities, including:
- Subduing the world and bringing it under our control
- Ruling over the world by exercising dominion over the creation
- Serving as caretakers of the planet
God has granted his creation to us. This responsibility entails that we use God’s creation and its abundant resources so that human beings can flourish. Yet human flourishing should not include a wanton disregard for the world in which we live. We are to exercise care for the planet and the life that inhabits it.
These mandates provide the motivation for science and technology. Fulfilling each of these responsibilities requires that humanity understands the world. That insight, in turn, should be used to develop technologies that allow humanity to thrive while ensuring the planet’s health and protecting Earth’s ecosystems.
Because we have dominion over creation, we are free to engage in biotechnology and synthetic biology, including projects such as the creation of lab meat. Creating and using lab meat to satisfy the growing worldwide demand for meat is not outside of God’s will or design. Advances such as these are exactly the very thing God expects of us as rulers over his creation. When we create lab meat, we are exercising the authority over creation that he granted us.
We also have an obligation as Christians to vigorously explore the potential of lab meat production to mitigate harm to the environment and to improve animal welfare. We also have an obligation to ensure that those who might be economically harmed by this technology are not marginalized, but protected.
Assuming that we can overcome all obstacles, then lab-grown meat may be an ethically viable alternative to animal agriculture. I don’t know about you, but I would be willing to serve lab meat at my Thanksgiving table one day in the near future. And I can’t help but wonder what John Madden would think of a turducken dish made from meat cultured in the lab.
Resources
Should We Play God?
“A Theology for Synthetic Biology, Part 1” by Fazale Rana (article)
“A Theology for Synthetic Biology, Part 2” by Fazale Rana (article)
“God’s Providence, Man’s Dominion, and Synthetic Biology” by Fazale Rana (article)
Lab Meat
Stars, Cells, and God: Lab Meat and Photosynthetic Zones by Fazale Rana and Hugh Ross (video)
Endnotes
- Martin Armstrong, “The Growing Global Hunger for Meat,” Statista (July 3, 2023).
- Alison George, “Lab-Grown Meat,” New Scientist, accessed August 24, 2023.
- Isam T. Kadim et al., “Cultured Meat from Muscle Cells: A Review of Challenges and Prospects,” Journal of Integrative Agriculture 14, no. 2 (2015): 222–233, doi:10.1016/S2095-3119(14)60881-9; Tom Ben-Arye and Shulamit Levenberg, “Tissue Engineering for Clean Meat Production,” Frontiers in Sustainable Food Systems 3 (2019): 46, doi:10.3389/fsufs.2019.00046.
- Lea Melzener et al., “Cultured Beef: From Small Biopsy to Substantial Quantity,” Journal of the Science of Food and Agriculture 101, no. 1 (January 15, 2021): 7–14, doi:10.1002/jsfa.10663.
- Tom Brennan et al., “Cultivated Meat: Out of the Lab, into the Frying Pan,” The Mackenzie Report (June 2021); Lana Bandoim, “Making Meat Affordable: Progress Since the $330,000 Lab-Grown Burger,” Forbes (March 8, 2022).
- Allison Aubrey, “‘No Kill’ Meat, Grown from Animal Cells, Is Now Approved for Sale in the U.S.,” NPR (June 21, 2023).
- Sghaier Chriki and Jean-François Hocquette, “The Myth of Cultured Meat: A Review,” Frontiers in Nutrition 7 (February 7, 2020): 7, doi:10.3389/fnut.2020.00007.
- Grace Galler, “What Do US Chefs Think of Cultured Meat?,” New Food (October 28, 2022).
- John Lynch and Raymond Pierrehumbert, “Climate Impacts of Cultured Meat and Beef Cattle,” Frontiers in Sustainable Food Systems 3 (February 19, 2019): 5, doi:10.3389/fsufs.2019.00005.
- Derrick Risner et al., “Environmental Impacts of Cultured Meat: A Cradle-to-Gate Life Cycle Assessment,” bioRxiv(April 21, 2023), doi:10.1101/2023.04.21.537778.
- For example, see Tobias Messmer et al., “A Serum-Free Media Formulation for Cultured Meat Production Supports Bovine Satellite Cell Differentiation in the Absence of Serum Starvation,” Nature Food 3 (January 2022): 74–85, doi:10.1038/s43016-021-00419-1.