Helpful Information in Understanding SARS-CoV-2 and COVID-19
Update on the COVID-19 Pandemic, March 17, 2020
Conditions change rapidly with a viral outbreak, and people sometimes wonder where to find good sources of information. To that end, I hope that a review of pertinent scientific data combined with personal encouragement will help you and those close to you be as informed as possible.
My Professional Background
I worked with a small team of dedicated scientists studying SARS-CoV at the National Institutes of Health (NIH) during the SARS (severe acute respiratory syndrome) outbreak of 2003. In my three years (2003–06) at the NIH we completed more than 50 projects that involved developing animal models for studying SARS pathogenesis and testing a variety of potential vaccines and treatments for SARS. We were successful in all these studies. And although we identified several good candidates for vaccines and prophylaxis, none were tested in human trials because SARS disappeared as fast as it appeared.
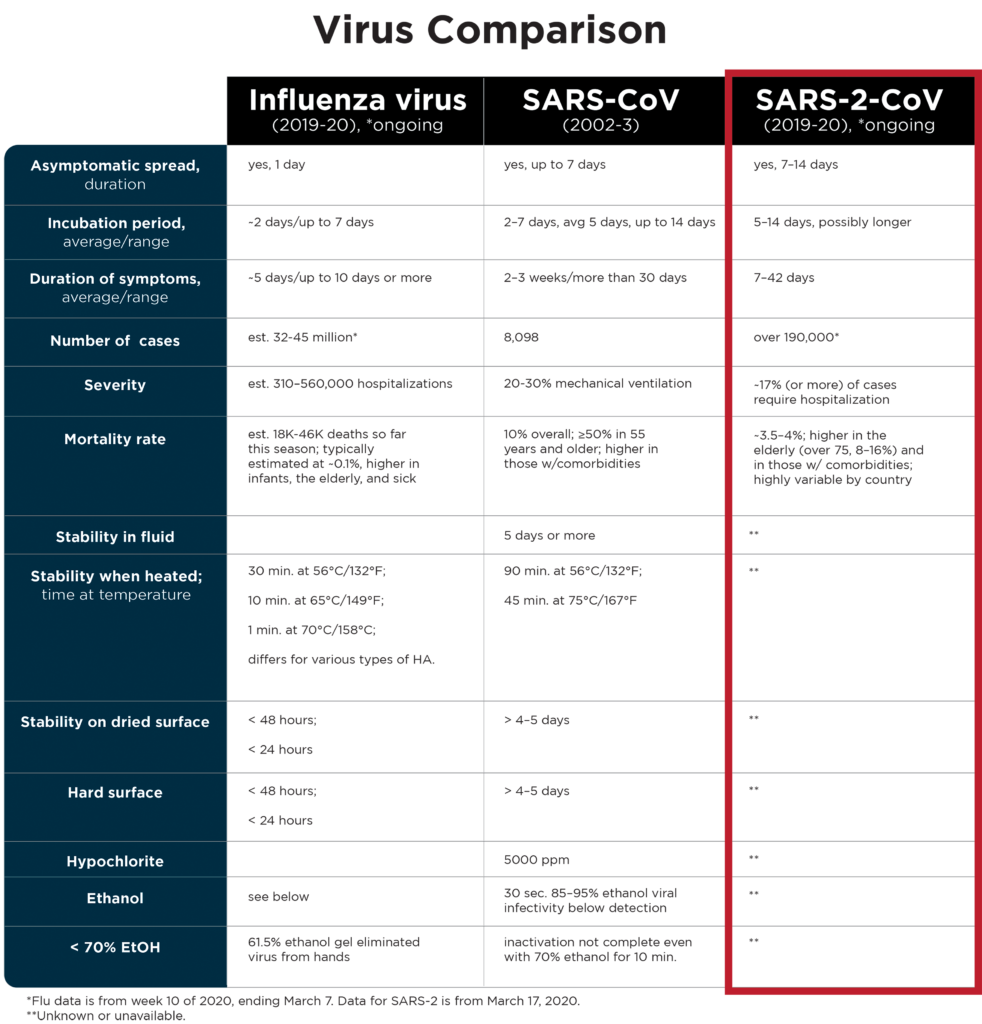
Viral Comparisons
There are a lot of similarities between the SARS virus and the novel coronavirus (nCOV-2019) that everyone is talking about. The similarities are such that this new virus has been officially named SARS-CoV-2 (hereafter SARS-2). The disease it causes has been named COVID-19 (coronavirus infectious disease 2019).
But there are also important differences between SARS-2 and SARS, other coronaviruses, and other viruses like influenza A and B (flu).
Both SARS-2 and SARS are coronaviruses—the name given to a family of viruses with shared genetic and structural characteristics. Coronavirus genomes are comprised of RNA, and as far as RNA viruses go, coronaviruses are among the largest. SARS virus particles measure 150–200 nanometers (nm) in diameter and the viral genome is roughly 30,000 bases. Structurally, the surfaces of these tiny virus particles look a little like a crown with viral proteins (spikes) protruding from the cell-derived membrane that surrounds the viral nucleocapsids. The spike proteins are what the virus uses to attach to cells, enter cells, and initiate infection.
Some Viral History
Before SARS the only known coronaviruses that infected humans (HCoV-229E and -OC43) were both associated with cold-like symptoms and upper respiratory infections. Coronaviruses, including those that infect other animals, are frequently associated with respiratory and/or gastrointestinal symptoms. Unlike these other coronaviruses, SARS was a surprise because the severe atypical pneumonia that resulted from infection led to death in about 10% of cases, and 20–30% of SARS-infected individuals required mechanical ventilation. From November 2002 through July 2003, SARS spread to 27 countries, infecting nearly 8,100 people and killing 774. The fatality rate was much higher for the elderly (over the age of 50, the risk of severe disease and death increased with each decade of life) and for those who had additional, underlying diseases such as diabetes mellitus, hypertension, and heart or kidney disease.
Following the SARS outbreak, screening for additional coronaviruses in patients with respiratory illness led to the discovery of two other coronaviruses circulating in the human population (NL63 and HKU1). NL63, HKU1, OC43, and 229E coronaviruses account for 10–30% of upper respiratory infections in adults.
In 2012 there was another coronavirus outbreak in the Arabian Peninsula. The virus, like SARS, led to severe atypical types of pneumonia and was dubbed MERS (Middle Eastern respiratory syndrome) coronavirus. As of November 2019, the cumulative case count for MERS is 2,494 with 858 fatalities, yielding a mortality rate of about 35%. Again, higher mortality is seen in the elderly and in those with underlying conditions. Diabetes mellitus, hypertension, cardiac diseases, renal disease, and bronchial asthma have been the most frequent comorbid (simultaneous) disorders. Following MERS infection, 50–89% of patients require mechanical ventilation support.
SARS and MERS coronaviruses spread to the human population from animals harboring the virus. In the case of SARS, a weasel-like animal known as a civet cat or palm civet was the culprit. In MERS, contact with infected dromedary camels introduces the virus into the human population where it spreads poorly from person-to-person. The environmental reservoir for SARS-2 (COVID-19) virus has not been identified, but there is a very high sequence similarity to bat coronaviruses.
SARS-2 virus, like SARS and MERS coronaviruses, also has a higher mortality rate in the elderly and those with underlying disease. Unlike SARS and MERS, SARS-2 has a much lower overall mortality rate. Thus far (data from March 17, 2020) SARS-2 has infected over 190,000 people in over 55 countries in about 4 months’ time. The number of reported fatalities is just over 7,500, which coincides with an overall mortality rate of 3.5–4%. In some countries where the virus has been circulating for a few weeks, the mortality rates vary from 0.98% in South Korea to 7.7% in hard-hit Italy (these numbers were 0.64% and 2.5%, respectively, on March 2). It is still early in the pandemic, so global and individual country rates may change.
How Can You Help Prevent Spread of SARS-2?
The US travel restrictions put in place on February 2 almost certainly helped delay the spread of SARS-2 in the US. Calls for communities to practice social distancing and sheltering in place will also slow the spread. Limiting discretionary travel and meetings of large groups of people will also help slow the spread. These are the best practices for each of us and, along with practicing good personal and environmental hygiene, these will make huge differences in the ultimate outcome.
Our goal is to slow and limit the spread. This will help our health system infrastructure continue to manage influenza hospitalizations while limiting COVID cases. Flu season usually ends in April/May, so a few weeks’ delay and a slow community spread of COVID cases will improve outcomes. Slowing the spread will also help maintain resource availability for testing and treating the infected.
Facial masks are critical for health care personnel and most people in the general public do not use them properly. Rather than deplete a precious resource that will keep our health care system working for the benefit of us all, leave the masks for the professionals who need them. If you’re sick, you should be self-isolating and will not need a mask.
The best way to prevent the spread of SARS-2 (and flu) based on knowledge garnered from the 2003 SARS outbreak and subsequent studies on SARS and respiratory viruses includes the following:
- Frequently wash your hands with warm soapy water to a lather for 30 seconds.
- Avoid touching your face (mouth, nose, eyes).
- Use freshly prepared bleach, diluted 1:10 or 1:5 with water, to spray surfaces. Let air dry then wipe them down.
- Use hand sanitizers if washing your hands is not an option.
SARS-2 Is More Challenging Than SARS in Some Ways
SARS and SARS-2 have different incubation periods than flu. During the SARS outbreak most infected individuals demonstrated symptoms within 5 days of infection. Virus could be detected in SARS-infected individuals up to 30 days (rarely, even longer) post-infection. In contrast, the estimated incubation period for SARS-2 is up to 14 days and the recovery period is highly variable, from days up to weeks. In both cases, the viruses can be spread by infected individuals who are not showing signs of illness, but this seems to be more common with SARS-2 than it was for SARS.
Influenza viruses are in a different virus family than the coronaviruses and have smaller, segmented RNA genomes (about 14,000 bases) and smaller virus particles (about 100 nm in diameter). Influenza has an average incubation period of 2 days and it may be spread while asymptomatic between days 1 and 2 post-infection. Flu patients typically recover within 5–7 days.
SARS virus spreads through contact and respiratory droplets (generated by sneezing, coughing, and speaking) and was isolated from saliva, sweat, tears, urine, and feces. Influenza viruses are also spread by contact and respiratory droplets. Contact refers to coming in contact with virus deposited on surfaces of other items, such as door or refrigerator handles or elevator buttons or rails, and then touching your mouth, nose, or eyes. The potential for transmission by contact is linked to the virus’s ability to survive on contaminated surfaces. In regard to contact, SARS is more stable outside the body and on hard or dried surfaces than influenza viruses (see table).
The initial symptoms for SARS, SARS-2, and influenza viruses A and B are similar: cough, fever, and myalgia. Severe cases may progress to an acute respiratory distress syndrome, a severe type of pneumonia, resulting in hospitalizations and sometimes death.
Although SARS (and SARS-2) and influenza viruses may be spread by respiratory droplets and contact and may be difficult to distinguish from one another based on initial clinical symptoms, the viruses differ significantly from one another in very important ways. SARS (and due to greater biological similarity, possibly SARS-2) virus is much more durable in the environment than the influenza virus (see table below). These viruses also differ in their incubation periods, duration of illness, duration and means of viral shedding, and mortality rates. It is also likely that they vary in rates of transmission and in other factors affecting infectivity, transmission, tropism, pathogenicity, and recovery.
Precautions while Helping Others
Although a healthy adult under the age of 50 may experience very similar clinical symptoms if contracting SARS-2 or influenza virus, these viruses do not share the same biology, structure, or overall risks to the more vulnerable populations within our communities. Although the risk to many young healthy adults may not be as severe, they can become infected and transmit the virus to elderly individuals or to people with underlying risk factors. The latter two groups incur a greater risk of severe disease with SARS-2. All people need to take precautions to protect the most vulnerable among us. For Christians, it’s one way we can serve one another in love in the name of Christ Jesus.
Finally, I encourage you to meditate on scriptures such as Psalm 139, which reminds us that God is intimately acquainted with all our actions and is with us whatever we face. Other scriptures remind us of the call to love our neighbors even at great personal cost. I am always challenged when I remember that we are called to show mercy especially to the most marginalized individuals in society. In this context, part of that call entails that we take personal precautions while not abandoning those who are in greater need than we are. Love boldly and pray as you serve others in these challenging days.
Resources
- A recent study of risk to younger adults: https://www.cdc.gov/mmwr/volumes/69/wr/pdfs/mm6912e2-H.pdf
- One study on environmental stability of SARS-2: https://www.nejm.org/doi/pdf/10.1056/NEJMc2004973?articleTools=true
Online source materials available at:
- World Health Organization, “Middle East Respiratory Syndrome Coronavirus (MERS-CoV),” November 2019, https://www.who.int/emergencies/mers-cov/en/.
- World Health Organization, “Emergencies Preparedness, Response: Summary of Probable SARS Cases with Onset of Illness from 1 November 2002 to 31 July 2003,” revised September 2003, https://www.who.int/csr/sars/country/table2003_09_23/en/.
- SR Weiss and S. Navas-Martin, “Coronavirus Pathogenesis and the Emerging Pathogen Severe Acute Respiratory Syndrome Coronavirus,” Microbiology and Molecular Biology Reviews, 69, no. 4 (December 2005): 635–64, https://www.ncbi.nlm.nih.gov/pubmed/16339739.
- “Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University,” accessed March 18, 2020, https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html?fbclid=IwAR1dcZxxg-3MZOWDYpQwn-4hEHUVs1WgPhFduklvjzZdAf6lvAESmUlKuIk#/bda7594740fd40299423467b48e9ecf6.
- Centers for Disease Control and Prevention, “2019–2020 U.S. Flu Season: Preliminary Burden Estimates,” March 7, 2020, https://www.cdc.gov/flu/about/burden/preliminary-in-season-estimates.htm.
References that populate the data table:
- B. Bean et al., “Survival of Influenza Viruses on Environmental Surfaces,” The Journal of Infectious Diseases, vol. 146, no. 1 (July 1982): 47–51, https://doi.org/10.1093/infdis/146.1.47.
- Jane S. Greatorex et al., “Survival of Influenza A(H1N1) on Materials Found in Households: Implications for Infection Control,” PLoS One 6, no. 11, (November 22, 2011):e27932, doi:10.1371/journal.pone.0027932.
- M. Lindsay Grayson et al., “Efficacy of Soap and Water and Alcohol-Based Hand-Rub Preparations against Live H1N1 Influenza Virus on the Hands of Human Volunteers.” Clinical Infectious Diseases 48, no. 3 (February 1, 2009): 285–91, https://doi.org/10.1086/595845.
- Shumei Zou et al., “Inactivation of the Novel Avian Influenza A (H7N9) Virus under Physical Conditions or Chemical Agents Treatment,” Virology Journal 10, no. 289 (September 15, 2013), doi:10.1186/1743-422X-10-289.
- G. Kampf et al., “Persistence of Coronaviruses on Inanimate Surfaces and Their Inactivation with Biocidal Agents,” Journal of Hospital Infection 104, no. 3 (March 2020): 246–51, https://www.journalofhospitalinfection.com/article/S0195-6701(20)30046-3/fulltext.
- Chloé Geller, Mihayl Varbanov, and Raphaël E. Duval, “Human coronaviruses: Insights into Environmental Resistance and Its Influence on the Development of New Antiseptic Strategies,” Viruses 4, no. 11 (November 12, 2012): 3044–68, doi:10.3390/v4113044.
- Anne-Marie Pagat et al., “Evaluation of SARS-Coronavirus Decontamination Procedures,” Applied Biosafety 12 no. 2 (June 1, 2007): 100–08, https://doi.org/10.1177/153567600701200206.
- SM Duan et al., “Stability of SARS Coronavirus in Human Specimens and Environment and Its Sensitivity to Heating and UV Irradiation,” Biomedical and Environmental Sciences 16, no. 3 (September 2003): 246–55, https://www.ncbi.nlm.nih.gov/pubmed/14631830.