Water: Designed for Life, Part 4 (of 7)
Guest writers John Millam, Ken Klos, and Iain D. Sommerville return with their series on the uniqueness of water. Up to this point, the authors have been describing a general theoretical basis for understanding water’s life-essential properties. Here in part 4, the authors turn our attention to specific ways those properties are ideally designed for life.
From a chemical viewpoint, the most fascinating thing about water is that it is unique. In many of its properties, water differs from similar compounds and has values outside the normal range. It is just these differences that make water so ideally suited for its role in the earth’s life support system.1
Last month we began our exploration of water by highlighting the water molecule’s bent shape (part 1) and the importance of hydrogen bonding (parts 2 and 3). Using this foundation, we can now discuss water’s specific properties. Parts 4 and 5 will focus exclusively on water’s thermal characteristics (those dealing with heat and temperature); part 6 will cover the remaining properties and part 7 will discuss the availability of liquid water in our solar system.
Water’s Exceptional Thermal Properties
In 1913, Lawrence Joseph Henderson published the first study demonstrating quantitatively that water’s properties, including its thermal characteristics, are distinctly beneficial for life.2 Among them are water’s high melting and boiling points (part 2) and expansion upon freezing (part 3); others include,3
- the second-highest heat capacity of all liquids and solids;
- the highest heat of evaporation of any known substance;
- the second-highest heat of melting of all known liquids; and
- the highest thermal conductivity of all known liquids.
To help those unfamiliar with these four properties, we will define each one in the context of its role in moderating global climate.
Heat Capacity
Heat capacity is a measure of a substance’s ability to absorb heat. Specifically, it is the amount of heat energy required to raise the temperature of a substance by one degree Celsius. The higher a substance’s heat capacity, the more heat the substance can absorb per degree of temperature increase. To put it another way, for a given amount of heat absorbed, compounds with higher heat capacities will experience a correspondingly smaller increase in temperature.
Thus, water’s high heat capacity insures that water temperatures remain stable and don’t change too rapidly—making water supremely suitable for global climate stabilization. If water were like most compounds (having a much smaller heat capacity), the result would be more-extreme differences in water temperature between summer and winter and between day and night—causing more-severe weather disasters.
This high heat capacity also enables water to store and transport enormous quantities of heat. For example, when oceans absorb heat, the ocean currents help to redistribute it (see figure 1). The currents carry warm water away from the equatorial regions and deliver it to the colder regions near the poles.4 Having released their heat, the cold return currents help cool the equatorial regions. This heat transfer process, dependent on water’s high heat capacity for effectiveness, results in a more even distribution of heat, reducing temperature differences around the globe.
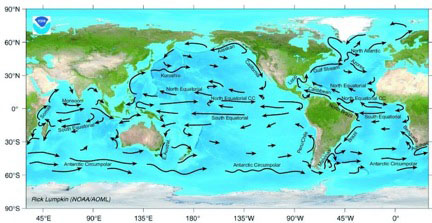
Figure 1. Surface ocean currents
Image credit: NOAA
Ocean currents also play a role in moderating land temperatures, particularly for coastal regions. For example, thanks to the Atlantic Ocean’s Gulf Stream, northern Europe (most notably, England, Ireland, and Iceland) is considerably warmer than other regions at similar latitudes.
Heat of Melting
The heat of melting is a measure of the heat needed to melt something. This measurement is also known as the heat of fusion because it simultaneously governs the reverse process (converting liquid into a solid). Water’s exceptionally high heat of melting helps protect Earth’s glaciers from melting. It also keeps oceans and most lakes from freezing completely. Both features are critical for aquatic life.
As water cools, its temperature decreases until it reaches its freezing point (0°C or 32°F for pure water). Further cooling, however, will not lower the temperature until all of the water freezes. Because oceans and large lakes are too big to freeze completely solid, their temperatures will never drop below water’s melting point. As noted in part 2, water’s melting point is exceptionally high. These two factors combine to ensure that oceans and most lakes never become too cold to support aquatic life.
Heat of Evaporation
The complement of the heat of melting (but at the other end of the temperature spectrum) is the heat of vaporization. Vaporization occurs when a liquid reaches its boiling point and changes from a liquid to a gas. Though it is the same process, evaporation can occur at any temperature (albeit at a much slower rate at lower temperatures). Thus, the heat of evaporation is the same as the heat of vaporization.
Evaporation plays a critical role in preventing lakes and oceans from becoming too hot. Sunlight imparts a lot of heat to water and, consequently, raises its temperature. Without a mechanism to shed most of this heat, many (perhaps all) bodies of water would become too hot for most marine life.
Earth avoids this hellish fate because of evaporative cooling’s efficiency. A portion of the liquid water is converted to water vapor, which carries away a lot of heat and cools the remaining water. To put this phenomenon in perspective, consider that the tropical oceans lose an estimated 2.3 meters of water per year due to evaporation—which amounts to the absorption of about 1,000,000,000,000,000 (one quadrillion) calories per square kilometer (or about 2.6 quadrillion calories per square mile)!5 In the case of lakes, evaporative cooling typically restricts the actual daytime temperature increase to only about 0.6°C (1°F) per hour.6
Water’s high heat of evaporation also provides another mechanism for moderating global temperatures. Evaporative cooling in the tropics results in warm, moist air. Similar to ocean currents, wind transports that air to higher latitudes to the north and south, where it helps warm colder climates. This transfer of heat from hot to cold regions helps balance world temperatures. Hence, without evaporative cooling, planetary temperatures would be subject to far greater changes.
Heat Conduction
Water is a remarkably good conductor of heat. In fact, its thermal conductivity is the highest of all liquids. A high thermal conductivity means that, rather than building up in a single location, excess heat will spread out quickly and distribute itself evenly. This characteristic of water is yet another means of moderating global temperature. Rather than heating or cooling only the surface, water moderates temperatures throughout the body of water by readily transmitting the heat it absorbs or loses to subsurface water. Therefore, raising the surface water temperature requires more heat and lowering them requires losing more heat.
Curiously, while liquid water is an excellent conductor of heat, solid ice is a thermal insulator. When ice forms over the surface of lakes, it traps heat like a thick coat or blanket, thus protecting the lake from further cooling (see part 3). Ice’s effectiveness as a thermal insulator also makes water ideal for supporting life.
Conclusion
Even this brief survey is sufficient to demonstrate the truth of the opening quote—water is the ideal substance for maintaining a planetary climate suitable for sustaining life. And water is able to do this only because of its exceptional ability to moderate heat more efficiently than other liquids. Without water and its unique properties, the entire planet would be subject to far more-extreme and rapidly varying temperatures. These extremes certainly would have prevented Earth from hosting any kind of complex plant or animal life. Even microscopic life would have been significantly limited.
Though we described each property individually, it should be noted that these characteristics actually work in concert and their effects reinforce one another. For example, the air currents’ effectiveness in moderating temperatures depends on both the heat of evaporation and heat capacity. If even one of water’s thermal properties had been lower (as with other common liquids), then complex life would not have been possible here on Earth or elsewhere. (An extensive discussion of the ways these properties work together can be found elsewhere.7) Nothing else could substitute for water because no other substance even comes close to matching all of water’s amazing thermal properties, much less its other unique characteristics. Clearly, water is designed for life.
In part 5 we will discuss two additional ways water’s thermal properties benefit life, as identified in Henderson’s study.8
To access additional parts, please click on a link below:
Part 1 | Part 2 | Part 3 | Part 4 | Part 5 | Part 6 | Part 7

Dr. John Millam received his PhD in theoretical chemistry from Rice University in 1997, and currently serves as a programmer for Semichem in Kansas City.

Mr. Ken Klos received his MS in environmental studies from the University of Florida in 1971, and worked as an environmental/civil engineer for the state of Florida.
Dr. Iain D. Sommerville
Dr. Iain D. Sommerville received his PhD from the University of Strathclyde, Glasgow, Scotland in 1966, and currently serves as professor emeritus of materials science and engineering at the University of Toronto.
Endnotes
- J. C. Bailer Jr. et al., Chemistry, 2nd ed., (Orlando, FL: Academic Press, 1984), 422.
- Lawrence Joseph Henderson, The Fitness of the Environment (New York: MacMillan Company, 1913), 72–132.
- R. A. Horne, Marine Chemistry (New York: Wiley 1969), 15.
- Henderson, The Fitness of the Environment, 88–89, 182.
- Ibid.,101–102.
- Ibid., 98–101. The value of 0.6°C per hour was taken from measurements of Lough Derg (the second largest lake in Ireland) during a hot summer.
- Michael J. Denton, Nature’s Destiny (New York: The Free Press, 1998), 42–45.
- Henderson, The Fitness of the Environment, 105.





