Elemental Evidence of Earth’s Divine Design
The familiar adage “You can’t have too much of a good thing” doesn’t hold true for planet Earth.
Too much water, for example, or too much carbon would destroy Earth’s ability to support advanced life. On the other hand, too little of certain “bad” things, elements generally considered poisonous to life, would also ruin Earth’s chances to serve as a life site. Many of these elements must be present on Earth in “just right” quantities or we wouldn’t be here.
When Carl Sagan and others first claimed that among the billions and billions of stars in the universe we’d be sure to find millions (or more) of life sites, they gave the impression that our home is just an ordinary, not-really-unusual planet. The past few decades of research, however, have shown us just the opposite: in its array and abundances of various elements and compounds, Earth is, in fact, extraordinary. Given its size and distance from the Sun, Earth holds an unusual and unexpected quantity of virtually every element and compound researchers can measure and compare—and especially in the case of elements and compounds vital to the existence of advanced life.
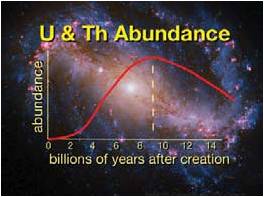
Figure 1: Cosmic Abundance history of Uranium and Thorium
Supernovae deliver uranium and thorium to the interstellar medium. However, the supernovae eruption rate declines as the universe ages. Eventually the delivery rate fails to keep pace with radiometric decay. For Earth to receive a maximal amount of uranium and t horium it must form when the cosmic abundance of those elements reaches a peak. (Credit for background image of the galaxy NGC 6217: NASA/ESA/Hubble SM4 ERO Team)
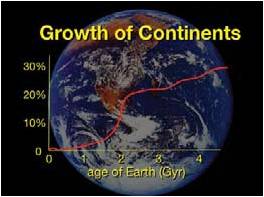
Astronomers have begun to gain some understanding as to how Earth came to be so different. It starts with the timing of the solar system’s origin. For example, as I often mention in my talks, our solar system formed at that moment in cosmic history when uranium and thorium reached their peak abundance (see Figure 1). Since that time, their production through supernova eruptions (the only cosmic source of uranium and thorium) has failed to keep pace with their radioactive decay. So their availability for incorporation into newly forming planets has decreased. The significance of this timing is that uranium and thorium are essential elements for driving Earth’s plate tectonics. Thanks to the timing of Earth’s formation and a number of subsequent events in the Sun’s and Earth’s early history (which will be described below), Earth was supplied with enough uranium and thorium to sustain strong, and enduring plate tectonics, which in turn provide for a just-right balance of oceans and continents—critical surface features for the recycling of nutrients and the existence of advanced life (see Figure 2).
Figure 2: Growth of Continents on Earth’s Surface:
The Vertical axis shows the percentage of Earth’s surface area comprised of continents. As the graph indicates, landmasses grew rapidly around the 2-billion-year mark and have increased gradually since then. The Current coverage of Earth’s surface by continents is ideal for the efficient recycling of nutrients and for the support of global human civilization. (Credit for background image of Earth: NASA)
The solar system formed not only with the best possible timing but also, simultaneously, in the best possible location for the sake of humanity. Both time and place influence the relative quantities of particular metal isotopes in early Earth’s composition. As British planetary astronomers Jamie Gilmour and Ceri Middleton noted, the exceptionally high ratio of aluminum-26 to aluminum-27 (4.5-5 x 105) at the solar system’s origin site is “difficult to produce in models of star formation.”1 In other words, it’s highly unusual and unexpected.
Four astronomers at the Universities of Hawaii and Colorado determined how the primordial solar system became so exceptionally enriched with aluminum-26.2 A prior generation of giant stars formed near enough to the giant molecular cloud from which the Sun and planets later emerged to shower it with aluminum-26 and other important isotopes (via “Wolf-Rayet” winds). If either the timing or location of the solar system’s formation were adjusted ever so slightly sooner or later or nearer or farther relative to these massive stars, the forming solar system would have been destroyed (or severely damaged) or would have been inadequately enriched with aluminum-26.
And yet without this high ratio of aluminum-26 to aluminum-27, there would have been no “thermal processing of planetesimals,”3 to use the words of Gilmour and Middleton. This would mean no heat pulse to drive off the dangerously high (for advanced life) quantity of volatile gases—carbon dioxide, carbon monoxide, nitrogen oxides, water, etc. from the primordial solar system. As the researchers pointed out, without this heat pulse Earth would have retained far too much water for continents to be possible and far too much carbon for an atmosphere to be breathable.
Meanwhile, to acquire sufficient quantities of other elements essential for advanced life, the emerging solar system had to form at the just right location and time relative to two different types of supernovae as well as to several objects called “asymptotic giant branch stars.”4
All the requirements described thus far mandate that the Sun and its planets begin to form in a large and dense star cluster. However, once it’s appropriately enriched with the uranium, thorium, aluminum-26, and other essential elements, the solar system must be ejected from this cluster because advanced life cannot tolerate close encounters with other stars.5
Earth, itself, demands special circumstances during its formative years. One of the most stunning was an event described in 2004 by Robin Canup. Her theory stated that a planet slightly more massive than Mars (Mars = 0.107 Earth masses) collided with the newly formed Earth—at an impact angle of about 45 degrees and impact velocity of less than 4 kilometers/second.6 This low-velocity collision brought about four significant-for-advanced-life changes: (1) it blasted away most of Earth’s water and atmosphere; (2) it ejected light element material and delivered heavy elements; (3) it transformed both the interior and exterior structure of Earth; and (4) it led to the formation of Earth’s exceptionally large Moon.7 (Canup improved her model in 2008, concluding that a somewhat larger collider impacted a retrograde rotating proto-Earth, while further confirming her original findings.)8
Approximately 650 million years after that collision, yet another pivotal (for advanced life) event occurred. An orbital resonance between Jupiter and Saturn disrupted the asteroids and comets of the Kuiper Belt, triggering an episode called the Late Heavy Bombardment9 (see Figure 3). Over a period of less than a hundred million years, some 17,000 impactors smashed into Earth, together depositing 200 tons of material on every square yard of Earth’s surface.10 Many of these impactors pierced through Earth’s crust. In the final analysis, this intense period of bombardment, as hellish as it may seem, brought about a chemical transformation, both interior and exterior, that favored the elemental needs of later advanced life.
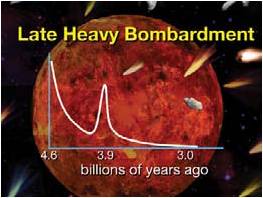
Figure 3: The Late Heavy Bombardment
Thousands of asteroids and comets pummeled Earth within a relatively brief time period some 3.9 billion years ago. This bombardment chemically transformed both Earth’s interior and exterior.
(Credit for images of asteroids and comets: NASA; credit for the deep space background: NASA/ESO; remaining artwork is the author’s.)
For a more complete list of Earth’s extraordinary quantities of chemical elements and compounds, see the table below. As you read it, be aware that every one of Earth’s exceptional abundances discovered to date has proved crucial for the support of life—advanced life, in particular. The evidence for the supernatural, super-intelligent, super-intentional design of Earth continues to mount.

Figure 4: Earth’s “Unobtainium,” Uraninite
Uraninite, also known as pitchblende, is the most uranium-rich ore on Earth. Uranium is more abundant on Earth than tin, antimony, or cadmium. Its exceptionally high abundance makes possible nuclear fission power plants. One kilogram of uranium-235 can yield up to 80 trillion joules of energy. And, because of its density, hardness, ease of use in machining and casting, and low cost, depleted uranium finds a wide range of industrial applications. [Photo Credit: Joachimsthal, Bohemia, Czechoslovakia Creative Commons (https://minerals.no-ip.com/Minerals/html/Pichblende1.html)]
Table: Earth’s Anomalous Abundances
The twenty-five elements listed below must exist on Earth in specific abundances for advanced life and/or support of civilization to be possible. For each listed element the number indicates how much more or less abundant it is, by mass, in Earth’s crust, relative to magnesium’s abundance, as compared to its average abundance in the rest of the Milky Way Galaxy, also relative to the element magnesium. Asterisks denote “vital poisons,” essential elements that if too abundant would be toxic to advanced life, but if too scarce would fail to provide the quantities of nutrients essential for advanced life. The water measure compares the amount of water in and on Earth relative to the minimum amount the best planet formation models would predict for a planet the mass of Earth orbiting a star identical to the Sun at the same distance from the Sun.11
| carbon* | 1,200 times less | cobalt* | 5 times less |
| nitrogen* | 2,400 times less | selenium* | 30 times less |
| fluorine* | 50 times more | yttrium | 50 times more |
| sodium* | 20 times more | zirconium | 130 times more |
| aluminum | 40 times more | niobium | 170 times more |
| phosphorus* | 4 times more12 | moybdenum* | 5 times more |
| sulfur* | 60 times less13 | tin* | 3 times more |
| potassium* | 90 times more | iodine* | 3 times more |
| calcium | 20 times more | gold | 5 times less |
| titanium | 65 times more | lead | 170 times more |
| vanadium* | 9 times more | uranium | 340 times more |
| chromium* | 5 times less | thorium | 610 times more |
| nickel* | 20 times less | water | 250 times less |
Endnotes
- J. D. Gilmour and C. A. Middleton, “Anthropic Selection of a Solar System with a High 26Al/27Al Ratio: Implications and a Possible Mechanism,” Icarus 201 (June 2009): 821–23. The quote is taken from the abstract.
- Eric Gaidos et al., “26Al and the Formation of the Solar System from a Molecular Cloud Contaminated by Wolf-Rayet Winds,” Astrophysical Journal 696 (May 10, 2009): 1854–63.
- J. D. Gilmour and C. A. Middleton, 821.
- Hugh Ross, “Solar System’s Extraordinary Birth Environment,” Today’s New Reason to Believe (February 16, 2009), https://www.reasons.org/astronomy/galaxy-design/solar-system’s-extraordinary-birth-environment.
- Juan José Jimenez-Torres and Barbara Pichardo, “Dynamics of Planetary Systems in Different Galactic Environments,” Astrobiology 8 (April 2008): 392; Hugh Ross, “Rare Solar System Location,” Today’s New Reason To Believe (October 20, 2008), https://www.reasons.org/rare-solar-system-location.
- Robin M. Canup, “Simulations of a Late Lunar-Forming Impact,” Icarus 168 (April 2004): 433–56; Robin M. Canup, “Dynamics of Lunar Formation,” Annual Review of Astronomy and Astrophysics 42 (September 2004): 441–75.
- Reasons To Believe’s documentary DVD Journey Toward Creation includes an animation clip and several illustrations that describe all four of these events.
- Robin M. Canup, “Lunar-Forming Collisions with Pre-Impact Rotation,” Icarus 196 (August 2008): 518–38.
- Richard A. Kerr, “Did Jupiter and Saturn Team Up To Pummel the Inner Solar System?” Science 306 (December 3, 2004): 1676.
- Ronny Schoenberg et al., “Tungsten Isotope Evidence From ~3.8-Gyr Metamorphosed Sediments for Early Meteorite Bombardment of the Earth,” Nature 418 (25 July 2002): 403–5; A. D. Anbar et al., “Extraterrestrial Iridium, Sediment Accumulation and the Habitability of the Early Earth’s Surface,” Journal of Geophysical Research 106, E2 (2001): 3219–36.
- Linda T. Elkins-Tanton and Sara Seager, “Ranges of Atmospheric Mass and Composition of Super-Earth Exoplanets,” Astrophysical Journal 685 (October 1, 2008): 1237–46; Hugh Ross, “Planet Formation: Problems with Water, Carbon, and Air,” Today’s New Reason To Believe (January 12, 2009), https://www.reasons.org/rtbs-creation-model/cosmic-design/planet-formation-problems-too-much-water-too-much-carbon-and-too-much-air.
- M. S. Sisodia, “Evidences Support an Extraordinary Event, Possibly an Impact During the Proterozoic for Phosphorus Abundance on the Earth,” Astrobiology 8 (April 2008): 360; Hugh Ross, “Where Did Earth Get Its Phosphorus?” (October 13, 2008), https://www.reasons.org/where-did-earth-get-its-phosphorus.
- Fabrice Gailard and Bruno Scaillet, “The Sulfur Content of Volcanic Gases on Mars,” Earth and Planetary Science Letters 279 (March 15, 2009): 34–43; Hugh Ross, “Sulfur-Poor Earth Conducive to Life,” Today’s New Reason To Believe (May 4, 2009), https://www.reasons.org/SulfurPoorEarthConducivetoLife; Benton Clark, “Death By Sulfur: Consequences of Ubiquitous S Before and After the Biotic Transition for Mars and Other S-Rich Planets,” Astrobiology 8 (April 2008): 433; Hugh Ross, “Too Much Sulfur,” Today’s New Reason To Believe (October 6, 2008), https://www.reasons.org/too-much-sulfur.