Song of the Intestine: So Kill Me, Maybe
Life and death in the intestine is all a matter of location, context, and company.
Location
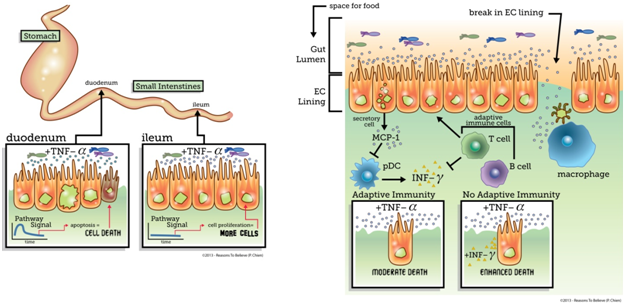
Human digestive systems perform a stunning array of tasks to break down food and absorb nutrients. Digestion is spatially and temporally coordinated across many organs to ensure that enzymes (which break down food) arrive as needed. Mucus along the gastrointestinal tract protects a single layer of cells (termed the epithelial cell [EC] lining) from the acidic enteric soup (ensuring you are not digested!) In this location, the intestinal lining, which is required to secure energy and other key nutrients, acts as the first line of defense against invading viruses, harmful bacteria, and parasites.
Not surprisingly, the EC lining––a true battle line––lives an intense, brief life, and its entire cell population is replaced every 4–5 days. Its multiple, diverse tasks require coordination with the immune system to ensure proper response to immunological threats and promote tissue homeostasis (stability). Though humans may be unaware, life and death are orchestrated through complex, myriad signals sent between intestinal cells and resident immune cells. The precise balance of life and death is critical for maintaining the integrity of EC lining and preventing chronic inflammation.
One such cell signal, TNF-α, is a pro-apoptotic (it stimulates programmed cell death) signal secreted by macrophages (cells that destroy foreign antigens), which have been activated by detection of bacterial invasion. Macrophages comprise part of the innate immune response, a set of fast-acting cells that respond to initial immunological challenges. Misregulation of TNF-α, can result in chronic inflammation and the development of a host of immunological issues (such as inflammatory bowel disease).
Context
Recently, researchers have used systems biology (the study of integrated dynamic systems) to probe signaling pathways and unravel mechanisms that allow precise coordination of life and death within the intestine.1 Scientists observed the fate responses of cells in two (of the three) different regions of the small intestine, the duodenum (upper) and ileum (lower), when doses of TNF-α were administered to mice. Surprisingly, while cells in the duodenum responded by undergoing apoptosis (programmed cell death), cells in the ileum responded by proliferating (cranking up growth). Analysis of the signaling networks indicated that the duodenum and ileum are wired for different dynamic responses to TNF-α, which reflect the fate choice. These results highlight the complex, context-dependent signal processing capabilities of cells even in the same organ. While the purpose of the differential responses to TNF-α are yet unclear, these results suggest that therapeutics targeted to the intestinal TNF-α pathway may have heterogeneous and unintended results. For example, a medical researcher might administer TNF-α to address cancer in the duodenum, but the same cell signal will exacerbate the disease in the ileum.
Company
In addition to the spatial regulation of fate in the intestine, the company cells keep also influences cellular decision-making. Researchers used a similar systems biology approach to unravel the interplay between immune cells and the EC lining in regulating intestinal homeostasis.2 Focusing on the duodenum, the researchers observed that in the absence of the adaptive immune system (T and B cells), where pathogens are “remembered,” programmed cell death was significantly enhanced. The researchers identified a protective signal, cytokine MCP-1, that was lacking in the absence of adaptive immunity. However, this signal was only weakly produced by immune cells, and the addition of MCP-1 in mice without adaptive immunity (no memory) did not reduce apoptosis. Instead, MCP-1 produced by the secretory cells appeared to block the migration of pro-apoptotic cells called pDCs. These pDCs secrete an additional pro-apoptotic signal called INFγ. Addition of INFγ with TNF-α resulted in an increase in cell death even in the presence of adaptive immunity, indicating adaptive immunity normally reduces death by modulating the secondary INFγ signal.
Researchers have not identified the mechanism for how the adaptive immune system modulates this response. The picture that emerges from the intestine is one of highly integrated, context-dependent signal relays that rely on orchestrated events coordinated across extracellular and intracellular mechanisms to maintain proper tissue homeostasis under varying conditions.
While such complexity and orchestration of a system does not necessarily preclude the possibility of an evolutionary mechanism, it demonstrates design across several orders of organization. Signaling must be coordinated in time to generate the precise response, and these events are mediated via dynamic wiring of signaling cascades encoded in the DNA. Beyond the temporal and molecular orchestration, events must be precisely ordered across at least seven different cell types, each playing a critical role in responding to immune threats. The elegant design of these systems and the myriad levels of orchestration add to the weight of evidence for design observed in biological systems.
–
Dr. Katie Galloway
Endnotes
- K. S. Lau et al., “In Vivo Systems Analysis Identifies Spatial and Temporal Aspects of the Modulation of TNF-α-Induced Apoptosis and Proliferation by MAPKs.” Sci Signal 4, no. 165 (March 2011): ra16.
- K. S. Lau et al., “Multi-Scale in Vivo Systems Analysis Reveals the Influence of Immune Cells on Tnf-Alpha-Induced Apoptosis in the Intestinal Epithelium.” [In eng]. PLoS Biol 10, no. 9 (September 2012): e1001393.